-
11 July 2025 | Policy Analysis
Steps to improve the accessibility and sustainability of non-medical health professions -
11 July 2025 | Country Update
Amendment of the Act on Health Services: Enhancing emergency care and patient rights, and reducing bureaucracy -
11 July 2025 | Country Update
Advances in vaccine reimbursement and the National Vaccination Strategy -
19 June 2025 | Policy Analysis
Major amendments to public health insurance and ehealth legislation -
09 November 2024 | Country Update
Amendment to the Act on Specific Health Services and other regulations -
06 September 2024 | Country Update
Definition of Telemedicine in Czech legislation -
08 June 2023 | Country Update
Measures to promote resilience in pharmaceuticals -
31 January 2022 | Policy Analysis
Change in the reimbursement process for highly innovative pharmaceuticals and orphan drugs -
31 May 2021 | Country Update
Changes in the process of how medical devices and aids enter the Czech market
2.7. Regulation
With a system based on compulsory SHI, the organizational relationship between HIFs and providers is based on long-term contracts. In terms of regulation, the main actors for the health system are the Czech Parliament, which sets the legal regulatory framework (including the basic benefits package, as detailed by legislative acts), and MZČR and MFČR, which jointly oversee HIFs. SÚKL and MZČR have regulatory roles for pharmaceuticals and MDA, while regional authorities register and supervise outpatient providers and inpatient providers other than those operated by the state. Inpatient facilities directly subordinated to the Ministries of Health or Defence are directly overseen by these ministries. MZČR is also responsible for licensing health professionals.Amendments to two laws – on public health insurance and electronic healthcare – passed the Czech Senate in June 2025. Both amendments now await the President’s signature and are expected to come into effect in January 2026.
Act on Public Health Insurance
According to the Minister of Health, the amendment aims to enhance the efficiency of healthcare financing, strengthen the role of health insurance funds and promote preventive care, with the overall goal of improving access to healthcare.
A key change is the restructuring of mandatory accounts within health insurance funds. The amendment abolishes reserve accounts and introduces a new account for public benefit activities (Fond obecně prospěšných činností), which will support initiatives aimed at improving healthcare quality. This includes partial financing of physicians’ specialty training, DRG activities conducted by the Institute of Health Information and Statistics (ÚZIS), and selected activities of patient organizations. Notably, health insurance funds will now be able to support residency training positions in specific regions or specialties, a role previously limited to the Ministry of Health, which is required to offer equal conditions nationwide.
The amendment also increases the permissible allocation for health promotion programmes from 0.5% to up to 3% of collected premiums. It intends to expand the possibilities of health insurance funds to offer benefits to insured individuals who take good care of their health. However, insurance funds may only access the increased budget if their financial situation is balanced.
Further changes include the possibility of receiving covered health services abroad up to the amount of local reimbursement if these services are unavailable in Czechia or if it is more efficient for the health insurance fund. This concerns both long-term and repeated use of care. Health insurance funds will be allowed to contract directly with foreign providers for such care.
Reimbursement for medical devices will now fall under a separate Act on the Categorization of Medical Devices, aimed at ensuring more flexible and timely responses to technological advances and evolving patient needs.
The amendment also aims to increase the accessibility of dental care. It responds to the ban on the use of amalgam dental fillings. Insurance funds will therefore reimburse their adult insured persons for the cheapest available white filling, or partially reimburse a higher-quality alternative.
Other passed changes include a report on the network of contractual providers of outpatient care in the fields or services specified in the governmental regulation on the local and temporal accessibility of health services (with the exception of pharmacies) and home care. This report will be published annually, by the end of November, by each health insurance fund. Furthermore, the health insurance funds will have the opportunity of centralized procurement for supplies of “centre” medicinal products for highly specialized providers. Also, the selection procedures at the regional offices before concluding a contract with the insurance fund will be eliminated. Decisions to contract a new provider will rest solely with the insurance funds.
Health insurance funds criticized the abolishing of the reserve account and the obligation to finance the management of the DRG system. The Ministry of Health countered by saying that the reserve accounts were not used in the past, even when the specific situations which the reserve accounts were established for occurred.
Furthermore, following substantial criticism, a proposed clause that would have allowed health insurance funds to seek (partial) reimbursement for care provided to individuals injured while committing illegal activities was removed from the final version.
Act on Electronic Healthcare
This amendment seeks to improve communication across the healthcare system and promote safer, higher-quality service delivery through digital tools.
The instruments introduced include:
- Electronic vaccination card
- e-Referrals
- Central register of preventive examinations
- Electronic vouchers for medical supplies (for example, crutches, bandages)
The “core” register is expected to be expanded to include, for example, data on medical fitness to drive motor vehicles, possession of weapons or ammunition permits. The shared health record will be divided into two parts – an emergency record with key data (for example, blood type, allergies) and a record of the results of preventive and screening examinations.
Medical check-up requirements for older drivers will also change. Instead of beginning at age 65, mandatory check-ups will now start at age 70. Drivers will no longer need to carry physical proof of these check-ups; police will verify them via digital records.
References
https://www.senat.cz/xqw/xervlet/pssenat/historie?cid=pssenat_historie.pHistorieTisku.list&forEach.action=detail&forEach.value=s5450 (Senate Press No. 103)
Authors
References
2.7.1. Regulation and governance of third-party payers
HIFs operate as quasi-public, self-governing bodies that collect SHI contributions and purchase health services. The state, through MZČR and MFČR, plays key roles in the regulation and governance of HIFs. Both ministries also have representation on the boards of trustees (správní rada) of HIFs and so have a say in managerial decisions.
HIFs are not permitted to make profits and are open to any applicant who is legally entitled to health insurance in Czechia; risk selection and cream-skimming are not permitted. Although all HIFs fundamentally serve the same purpose, there is a special law establishing and governing VZP (Act no. 551/1991 Coll.), while establishment of all other HIFs is from a different law (Act no. 280/1992 Coll.). VZP differs from the others regarding its role as an insurer of the last resort (in case of a HIF’s bankruptcy or insolvency) and because its solvency is explicitly guaranteed by the state.
VZP also differs from other HIFs in terms of its organizational structure and governance. There is a legal requirement to have 14 VZP regional branches, one in and for each Czech region. Though other HIFs are smaller in population share and/or geographical presence, they are free to expand if they so choose; most general practitioners (GPs), outpatient specialists and hospitals contract with all HIFs relevant in their region. The smallest HIF is the Škoda Employee Insurance Fund (Zaměstnanecká pojišťovna Škoda), insuring around 1.4% of the Czech population (nearly 150 000 members and open to all).
HIFs are managed by directors, who in turn are appointed by trustee boards. The boards provide oversight of their respective directors and the decisions they make, which can include calling on directors to change decisions or limiting their managerial authority. Furthermore, certain directorial decisions explicitly require prior board consent, as defined by law. In the case of VZP, the board has 30 members, 10 of whom are nominated by MZČR and appointed by the government; the other 20 are elected by the Chamber of Deputies in proportion to the parliamentary parties’ strength. Members of the board are not personally liable for decisions made by the board as a whole or for VZP’s performance.
For other HIFs, board compositions are based on a system of tripartite representation. One-third of board members are appointed by the government; another third of members are elected among employers contributing the largest shares of the given HIF’s collected contributions (usually from industry, but in some cases also from civil service); and the remaining third of the board members are elected representatives from the HIF’s insured members, usually from labour unions at firms with significant employer contributors to the respective HIF. The election procedures for the latter two groups legally changed in 2020, broadening the eligibility for employers to nominate candidates and for more participation for insured members outside labour unions, respectively. Altogether, these HIFs have 15 board members. Like their VZP counterparts, board members bear no personal liability for board decisions or for the HIF’s performance.
All HIFs also have supervisory boards (dozorčí rada) as their highest level of governance; the narrow scope of regulatory oversight means, however, that their roles are rather limited. Their main tasks are to ensure that HIFs follow internal rules and adhere to a set financial and operating plan. VZP’s supervisory board consists of 13 members, with MZČR, MFČR and the Ministry of Labour and Social Affairs (Ministerstvo práce a sociálních věcí, MPSV) each nominating one member to be appointed by the government, while the other 10 are elected by the Chamber of Deputies, again using a proportional method based on parliamentary representation. The supervisory boards of the other HIFs consist of nine members and are based on a system of tripartite representation similar to that used to constitute the trustee board. MZČR, MFČR and MPSV each nominate one member to be appointed by the government.
To ensure that HIFs are held accountable for their performance, they are obliged to submit their financial and operating plan for the next year every autumn, including the outlook for next two years ahead. After the plan is approved by a HIF’s board, it is submitted to MZČR, which reviews the document in collaboration with MFČR. Subsequently, the plan is sent for governmental approval and then submitted for final approval to the Chamber of Deputies. If the plan is not approved before the start of the following year, a HIF acts according to the submitted plan. A similar procedure is used for approving the final accounts and annual reports of HIFs. However, plan approval is rarely withheld, nor are they often amended, so main oversight and de facto approval lie with MZČR and MFČR.
On a quarterly basis, HIFs submit their financial reports and other requested information to MZČR and MFČR for review; they also carry out regular inspections and spot checks. If irregularities or errors are identified, MZČR may call for correction. In very serious cases, MZČR can place a HIF under forced administration or, as a measure of last resort, can revoke its operating licence. This may happen, for example, in cases of poor economic performance, if a HIF is in serious debt or cannot meet its liabilities, or because of failure to comply with the public interest. Members of a HIF whose licence has been revoked are automatically switched to VZP. Since the 1990s, there has been only one example of forced administration; VZP was put under forced administration for almost six months due to poor economic performance and large debts in 2005.
With regard to the HIFs’ internal accounting systems, MFČR publishes a directive that (1) specifies the different accounts that HIFs must create, and (2) limits transfers between these accounts so that, for instance, only a certain percentage of revenues can be spent on operating expenses. Examples of internal accounts include a reserve account; an account for financing health promotion programmes; an account for financing investments; an account to cover operating expenses; and, of course, an account for reimbursing providers for services. Health promotion accounts are used to reimburse members, such as for non-SHI-covered vaccinations and sporting activities, though this may differ among HIFs (see section 3.3.1).
Finally, to create a new HIF, applicants must apply for a licence from MZČR. During the licensing process, the application is reviewed by MZČR and MFČR. Both may request to review additional information or supporting documents. MZČR must decide on the application within 180 days of receiving it. If all conditions are fulfilled, the applicant is legally entitled to a licence; only legal entities residing in Czechia may apply. Applicants are required to set aside financial reserves (in the reserve account described previously) before being licensed; after a HIF is established, the reserve should function as a financial buffer in case of a temporary liquidity shortage. Within one year of establishment, a new HIF must prove that it has at least 50 000 insured members. Mergers of HIFs have to be approved by MZČR, which assesses that the merger is not disadvantageous to the existing system. Mergers occurred in the past either if one faced financial difficulties or in order to benefit from shared structures and increased efficiency; the last merger took place in 2012.
2.7.2. Regulation and governance of provision
The regional authorities are responsible for authorizing health service provision for outpatient providers as well as inpatient providers that are not directly subordinated to any ministry or region. That said, apart from teaching hospitals and state-owned specialized inpatient facilities, and regional hospitals that were not transformed into commercial companies (regional hospitals that remained directly subordinated, so-called budgetary organizations of a region), all other providers must be authorized by their respective regional authority in order to provide services (see Table2.1).
Table2.1
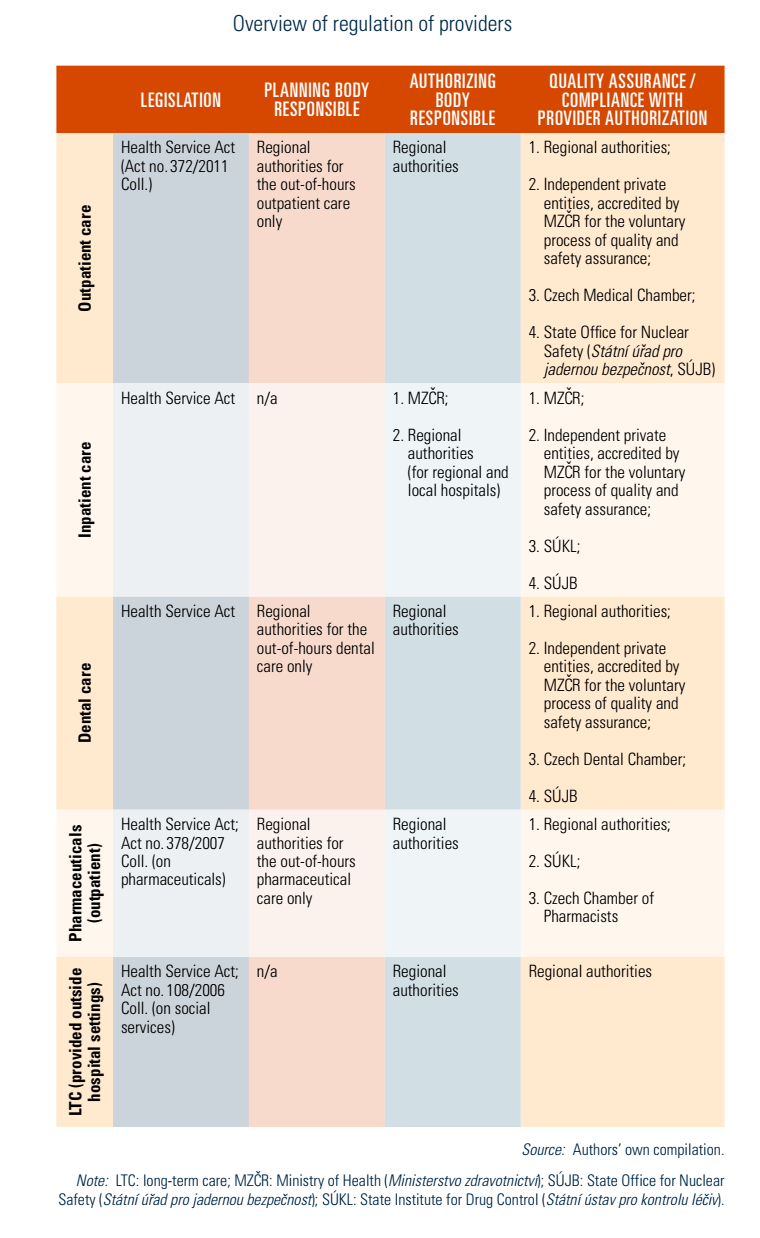
As part of the authorization process, the type and scale of services that providers are permitted to provide are defined. Since 2012, the minimal staffing and technical requirements are legally defined and binding for all providers (Health Service Act, 2011); providers must also comply with hygiene requirements, among others. Any changes to the information provided during the authorization process, including a change in professional staff, must be reported to the respective regional authority. In addition, providers must report any change that occurs regarding the scope of their authorization to the National Register of Healthcare Providers (for example, places of provision or operating hours).
Upon completing the authorization process, a provider may either (1) start its operations thereafter by taking out-of-pocket (OOP) payments (seen in dental care or cosmetic surgery) or (2) try to conclude a contract with HIFs under the condition that a mandatory selection process, organized by MZČR for inpatient providers and by the regions for outpatient providers, establishes a proof of need for the new provider or for the extended services of an existing provider. Concluded contracts may not always be identical to a provider’s full range of authorized services, meaning that providers may offer other (but authorized) services for OOP payments. Additionally, a contract with one HIF does not guarantee contracts with all HIFs. HIFs cannot sign new contracts without the above-mentioned selection procedure taking place and establishing the proof of need.
MZČR is also responsible for licensing health professionals, including physicians, dentists, nurses, pharmacists and paramedics. Licensing procedures consider applicants’ professional qualifications along with performance on standardized state licensing examinations (státní atestační zkouška). For more information on training and licensing of health professionals, see section 4.2. To open a private practice, licensed physicians must apply for authorization from their respective regional authority, as described above.
All inpatient providers are legally obliged to have an internal system in place for monitoring quality, though no external authority collects or reviews the results. MZČR offers some guidance regarding the scope and organization of internal quality monitoring, but providers are free to choose how to do it.
There is no system-wide compulsory accreditation system for quality standards. Accreditation process for providers is voluntary. However, it can only be performed by an accredited third party that fulfils the legal requirements of independence and professional background and staffing; MZČR grants these third-party accreditations. Any organization fulfilling the accreditation requirements can apply; these criteria were created over the past decade to standardize accreditation organizations.
To define networks that ensure the delivery, accessibility, proper utilization and staffing of specialized treatment, MZČR can assign inpatient providers the status of being highly specialized health care centres; this procedure is described in law (Health Service Act, 2011). MZČR publishes the list of highly specialized centres for each selected medical specialty in its decrees (see section 5.4.3). Centres are subject to quality indicator reporting and regular monitoring of their compliance with centre requirements as defined in the application process (Bryndová et al., 2021).
Since 2015, the Agency for Health Care Research of Czechia, a state agency directly subordinated to MZČR, is responsible for the development of clinical guidelines. The project is currently in its pilot phase, with 19 clinical guidelines developed and approved as of May 2022 and 24 more being prepared or consulted (KDP, 2022).
In July 2025, the Czech government launched a major investment programme to strengthen the education of non-medical health professionals, with CZK 12.8 billion (EUR 520 million) allocated between 2026 and 2037. The programme, developed jointly by the Ministry of Health, public universities and professional associations, aims to expand training capacity in key fields such as general nursing, paediatric nursing, paramedicine, midwifery, radiology assistance and nutritional therapy. It targets a minimum 20% increase in student intake to support long-term staffing sustainability in the healthcare sector. Funding will be directed towards infrastructure upgrades, recruitment of academic staff, and improvements in admissions processes and practical training. Additional measures include streamlining specialization training, enhancing mental health and career support for educators, and strengthening links between education and clinical practice. The initiative responds to structural changes in the Czech health workforce and aims to maintain access to quality care in the context of population ageing.
Furthermore, in July 2025, the Czech Senate approved an amendment to the Act on Non-Medical Health Professions, aiming to improve access to the professions of practical nurses and paramedics by adjusting education requirements and allowing earlier entry into clinical practice. The amendment now awaits presidential signature. Under the new rules, students of general nursing, paediatric nursing and paramedicine will be allowed to begin working in hospitals as practical nurses after completing four semesters of a bachelor’s programme or two years at a higher vocational school – shortened from the current six semesters or three years. Paramedic students will also be able to join the workforce after five semesters or 2.5 years of vocational training.
The reform is designed to alleviate staffing shortages in hospitals and provide students with earlier hands-on experience. It also simplifies training requirements for future paramedics by allowing mandatory clinical placements to be completed directly within emergency medical services. In addition, the amendment aligns Czech legislation with EU rules on qualification recognition, including more flexible requirements for Romanian nurses seeking employment in Czechia.
References
A major change is the transfer of responsibility for organizing emergency services (pohotovostní služby) from regions to health insurance funds, which is expected to improve equitable access by leveraging insurers’ greater capacity.
The reform also bolsters patient rights and protection by:
- introducing hospital ombudsmen to handle complaints directly within facilities,
- strengthening protections for patients at risk of domestic or sexual violence, and
- strengthening the right to transparent information about the cost of healthcare services and significantly tightening penalties for illegal fees charged by doctors.
The amendment also establishes centres for comprehensive care for children with long-term health conditions. These centres will provide coordinated, multidisciplinary care to improve the quality of life for paediatric patients and help families navigate the healthcare system more easily.
2.7.3. Regulation of services and goods
Basic benefits package
The range of benefits covered by SHI in Czechia is very broad (see section 3.3.1). A combination of means is used to define the basic benefits package; the most important of them is legislation, namely the Health Insurance Act (1997). Any changes in defining the benefits package, or to the positive and negative lists of benefits, require an approval from parliament and the president. Proposals are usually submitted by the government, though regions and members of both parliamentary chambers can propose new legislation or amendments. The five annexes to the Health Insurance Act define (1) negative lists (namely, explicitly excluded services) and (2) positive lists on pharmaceutical substances, MDA, dental aids and procedures, and a spa treatment indication list.
The principle of a basic benefits package being defined by law was put forward in a 2013 court decision abolishing the above-standard care co-payments (Alexa et al., 2015). Although the ruling found a system of above-standard procedures was legally sound in principle, the court took the view that a list of them must be specified by law and not, as was the case, only by governmental decree (Constitutional Court of the Czech Republic, 2013).
SÚKL is responsible for determining the list of pharmaceuticals covered by SHI and the depth of their coverage, which is done via reference pricing and Anatomic Therapeutic Chemical (ATC) groups for prescription pharmaceuticals (see section 2.7.4).
Benefits are also rationed by the LHS, a fee schedule known as the List of Health Services (Seznam zdravotních výkonů) that is issued as a directive from MZČR. The LHS is primarily intended for reimbursement purposes, but for everyday practice it serves as a positive list of benefits. The LHS is updated annually based on decisions taken within the Health Services Working Group; any stakeholder can propose modifications. In addition to the applicant who requests a change, the Working Group includes representatives of MZČR, ČLS JEP, the outpatient and inpatient providers’ associations (including for physicians and nurses), representatives of HIFs and a patient representative. The group meets on a continuous basis throughout the year and decides, based on a consensus procedure, which LHS items will be added, removed or modified. At the end of each year, the updated LHS is issued as a directive by MZČR. The last amendment to date, which amended MZČR Directive no. 134/1998 Coll., on the List of Health Services and Point Values (MZČR, 1998), resulted in the LHS containing 3995 items for 2022 (MZČR, 2021a).
Health technology assessment
There is no public body systematically conducting comprehensive analyses of gathered information to enable evidence-based policy approaches.
In 2021, health technology assessment was not used systematically in SHI coverage or reimbursement decisions, except for pharmaceuticals. Only some evidence-based criteria are taken into consideration when there are requests to change the LHS. For instance, applicants are required to submit a range of evidence that includes an assessment of efficacy, a comparison with existing treatments (if possible), a projection of expected costs to the SHI system, and a description of the mechanisms of reimbursement employed in foreign countries.
For the process of setting reimbursement rates for pharmaceuticals, SÚKL requires applicants to supply evidence of the clinical effectiveness and cost-effectiveness of a pharmaceutical as well as an analysis of the impact a positive reimbursement decision would have on the SHI system (SÚKL, 2020).
From 2026, vaccine reimbursement in Czechia will follow a more predictable and systematic process, removing the need for legislative amendments each time a new vaccine is introduced. This reform is expected to improve access to modern vaccines. Under the amended Act on Public Health Insurance, the State Institute for Drug Control (SÚKL) will determine the level and conditions of reimbursement for immunization-related medicinal products through a formal health technology assessment (HTA) process. HTA will consider medical, social, economic and ethical aspects, along with the broader public health impact of each vaccine.
To support better access and uptake of vaccines, the Ministry of Health also submitted a draft of the country’s first comprehensive National Vaccination Strategy for internal consultation in July 2025. The strategy addresses the full vaccination life cycle – from vaccine approval and reimbursement to communication with the public and healthcare professionals and evaluation of the immunization programme. Aiming to improve coordination, transparency and public trust in vaccination, the strategy is expected to be approved by the government by the end of 2025.
Authors
In November 2024, the government approved an amendment to the Act on Specific Health Services (No. 373/2011 Coll.) and other regulations. This motion now has to pass through the Chamber of Deputies and Senate (in both, the government coalition currently holds the majority) and be signed by the president to be effective.
Among the main changes to the Act on Specific Health Services is the cancellation of some mandatory medical examinations. The goal is to reduce the administrative burden of both general practitioners, bring financial savings from decreasing the necessity of medical documentation extracts, and bring time savings to all participants. The cancellation concerns:
- the assessment of medical fitness for high school education, unless health limitations exist for the programme,
- annual medical fitness certificates of non-professional athletes who, for example, attend training in a sports club once a week, and
- entrance occupational medical exams for professions in the first (“without-risks”) category; however, both the employer and the applicant may still require the entrance medical examination.
The amendment further develops the new concept for health promotion programmes: combining protection against occupational and civil risks. Voluntary health support programmes are proposed, the purpose of which are to motivate employees to undergo preventive examinations and take care of their health.
Other changes will occur in the areas of assisted reproduction, protective treatment, genetic examinations, among others.
In a proposed amendment to Act No. 258/2000 Coll., on the protection of public health, changes apply to the self-employed in Czechia. Those classified self-employed in the area of accommodation services and epidemiologically significant activities (for example, hairdressing, massages, pedicures, etc.) should no longer be obliged to apply for approval of their operating regulations by the regional public health authority.
Authors
2.7.4. Regulation and governance of pharmaceuticals
Regulation of pharmaceutical products
MZČR, the Ministry of Environment, SÚKL and SÚJB are responsible for the regulation and governance of pharmaceuticals.
MZČR approves and controls specific treatment programmes; regulates the use of non-registered pharmaceuticals (for example, within specific treatment programmes or in case of a threat to public health (Act no. 378/2007 Coll.)); takes part in the preparation of the European pharmacopoeia; defines the Czech pharmacopoeia that describes the parameters of pharmaceuticals production and manipulation; and controls and makes publicly available lists of individuals authorized to dispose of unused or expired pharmaceuticals.
The Ministry of Environment assesses pharmaceuticals containing genetically modified organisms and assesses impacts of pharmaceuticals on the environment. In the case of radiopharmaceuticals, SÚJB also takes part in the registration and clinical assessment.
SÚKL is the main regulatory body for pharmaceuticals. It is responsible for the supervision of properties of medicinal products for humans. SÚKL’s activities relate to monitoring of quality, safety and efficacy of pharmaceuticals in all stages of development, sale and use. For this purpose, SÚKL uses a system of preliminary reporting, authorization procedures, inspections, laboratory controls and monitoring of practical use of medicines. SÚKL is entitled to act when a risk to public health arises, to impose penalties and to request necessary documentation. Furthermore, SÚKL authorizes access for pharmaceuticals before their entry onto the Czech market. The market authorization procedure includes an assessment of a dossier, in which a prospective authorization holder describes the safety, efficacy and quality of the product. The indications, contraindications, dosage of the product, general classification for supply, and the package patient leaflet are also assessed. During the registration process, SÚKL classifies pharmaceuticals into one of the four categories: prescription only, prescription only with restriction (for example, opioids, cannabis for therapeutic purposes, abortion pills), without prescription and without prescription with restriction (for example, pseudoephedrine – restriction on quantity).
For prescription pharmaceuticals and those with restrictions, an electronic system from SÚKL is in place. Since January 2018, it is obligatory for all providers to issue prescriptions in electronic form. Since mid-2020, full patient records on prescribed pharmaceuticals are available to attending physicians and to pharmacists; a patient can deny access to their individual record for a particular provider or to all, or selectively allow access for some providers. The system also allows for polypharmacy checks and control of potential duplicate prescriptions.
SÚKL also identifies and sanctions illegal conduct; the European regulation on counterfeit pharmaceuticals, including the unique-per-package barcode monitoring, is fully established in Czechia. Activities requiring effective authorization and supervision by SÚKL include manufacturing, import, distribution, supply or sale, preparation and parallel import, performing clinical trials and reference laboratory activities.
In 2013, the surveillance activities of SÚKL were extended to narcotic and psychotropic substances. SÚKL is also charged with the surveillance of quality and safety of human tissues and cells intended for use in humans.
Regulation of wholesalers and pharmacies
Wholesalers need permission from SÚKL to distribute pharmaceuticals; SÚKL may fine them or suspend or cancel their permission to distribute pharmaceuticals. All pharmacies have to be registered by SÚKL and meet certain requirements on staff education and training. There is no limit on the number of pharmacies.
Generic substitution has been allowed in pharmacies since 2008, conditional upon the same active substance and the same mode of administration.
Mail-order and Internet pharmacies have to be listed by SÚKL, and sales are limited to over-the-counter pharmaceuticals without volume or frequency restrictions; pharmacies are responsible for ensuring safe delivery and providing pharmacists for consultations during office hours. At the time of writing, extending mail-order pharmacies to cover prescription pharmaceuticals has been under expert discussion, though not yet under serious legislative consideration.
System for pricing prescription pharmaceuticals
Since 2008, SÚKL has been responsible for determining the maximum prices of pharmaceuticals and for determining the level and conditions of reimbursement. Only the prices of pharmaceuticals covered by SHI are regulated and the price regulation is based on two mechanisms: (1) the maximum end-customer price – this is the average of the three lowest prices in the EU; and (2) a maximum trade margin determined by the so-called Price Decree from MZČR (usually a certain proportion of the ex-factory price).
The conditions for reimbursement are also regulated. Prices for prescription pharmaceuticals usually consist of SHI reimbursement and patient co-payment (which may be zero). A system of reference groups is in place, each group consisting of pharmaceuticals with similar effects and safety levels (considered substitutable at the beginning of treatment); generally, the reference groups are broader than ATC groups. According to the law, there must be at least one fully reimbursed pharmaceutical in each of the 195 existing groups. In case of need, SÚKL re-evaluates prices, and thus also reimbursement level, of prescription pharmaceuticals. For the process of setting reimbursement rates, SÚKL requires applicants to supply evidence of the clinical effectiveness and cost-effectiveness of a pharmaceutical, as well as an analysis of the impact a positive reimbursement decision would have on the SHI system (SÚKL, 2020)
There are annual limits on pharmaceutical co-payments for prescription pharmaceuticals, the actual price of which exceeds the reference price in a particular pharmaceutical group (however, not all co-payments count into the limit, only the level of co-payment of the least expensive alternative with the same active substance and the same mode of administration counts). Limits differ by groups of people, reflecting their socioeconomic vulnerability, and patients are reimbursed automatically on a quarterly basis by their HIF after reaching the annual limit (see section 3.4.1).
Since January 2022, changes to reimbursements of innovative medicines and orphan drugs have been implemented, opening up access to and availability of these products for Czech patients. For highly innovative medical products, the temporary reimbursement period has been extended from two years to three years for the first temporary reimbursement and from one year to two years for the second temporary reimbursement. However, the market authorization holder is obliged to reimburse HIFs for costs exceeding those indicated in the budget impact analysis that served as the basis of SÚKL’s decision and there is a follow-up treatment obligation in case the product is not accepted for permanent reimbursement after the five-year period. For orphan drugs, a new pathway gives registered patient organizations and medical societies a voice in the administrative procedure to set prices and reimbursement rates (see sections 2.8 and 7.1). Social impact and benefits have been newly added to extend the scope of evaluation criteria. In the case of orphan drugs, HIFs can also initiate reimbursement setting procedures; in these cases, market authorization holders are not obliged to pay back costs exceeding the budget impact analysis on which SÚKL based its issuance of the temporary reimbursement decision.
According to Act No. 378/2007 Coll., on pharmaceuticals (as amended), the State Institute for Drug Control (SÚKL) must be informed about all changes in medicines supplies (start, interruption, renewal, termination) by the registration holders who were permitted to supply medications to the Czech market. If notified of an interruption or termination of supplies of an irreplaceable medicinal product, SÚKL immediately reports this to the Ministry of Health.
In reaction to the lack of medication during winter 2022/2023 (especially of children’s antipyretics), multiple extraordinary measures were taken. A Working Group led by the Ministry of Health was established in January 2023 (further composed of representatives of SÚKL, the Czech Chamber of Pharmacists and pharmaceutical distributors). Over the winter, multiple emergency supplies of medicines were secured. Extra supplies were also ensured by issuing an extraordinary measure, where the Ministry of Health stipulates reimbursement of medicinal products registered in other EU countries for 12 months from 1 May 2023.
The Ministry proposed a systematic measure in the form of an amendment to Act No. 378/2007 Coll., on pharmaceuticals. This proposal was approved by the government in June 2023 and further legislative procedure will follow. It would oblige the registration holders to have a supply of medicines for one or two months. However, this is strongly opposed by the Chamber of Commerce and the Confederation of Industry in Czechia.
References
Act No. 378/2007 Coll., on pharmaceuticals (as amended)
Ministry of Health. Press release 4 January 2023. “Dnes zasedla k prvnímu jednání pracovní skupina k řešení nedostatku některých léčiv.” [Today, the working group dedicated to the shortage of certain medicines met for its first meeting.] https://www.mzcr.cz/tiskove-centrum-mz/dnes-zasedla-k-prvnimu-jednani-pracovni-skupina-k-reseni-nedostatku-nekterych-leciv
Ministry of Health. Press release 13 January 2023. “Do České republiky během ledna a února dorazí díky jednáním s výrobci a distributory téměř půl milionu balení antibiotik v různých skupinách.” [Thanks to negotiations with manufacturers and distributors, almost half a million packages of antibiotics in various groups will arrive in the Czech Republic during January and February.] https://www.mzcr.cz/tiskove-centrum-mz/do-ceske-republiky-behem-ledna-a-unora-dorazi-diky-jednanim-s-vyrobci-a-distributory-temer-pul-milionu-baleni-antibiotik-v-ruznych-skupinach
Ministry of Health. Press release 12 April 2023. “Vláda dala souhlas s mimořádným opatřením, kterým Ministerstvo zdravotnictví stanoví úhrady mimořádně dodaných antibiotických suspenzí pro děti, a to od 1. května 2023.” [The government has given its approval to an extraordinary measure by which the Ministry of Health determines the payment of exceptionally delivered antibiotic suspensions for children, starting on 1 May 2023.] https://www.mzcr.cz/tiskove-centrum-mz/vlada-dala-souhlas-s-mimoradnym-opatrenim-kterym-ministerstvo-zdravotnictvi-stanovi-uhrady-mimoradne-dodanych-antibiotickych-suspenzi-pro-deti-a-to-od-1-kvetna-2023
Ministry of Health. Press release 14 June 2023. “Vláda schválila novelu zákona o léčivech, která zásadně pomůže odolnosti českého lékového trhu.” [The government has approved an amendment to the Medicines Act, which will fundamentally help the resilience of the Czech pharmaceutical market.] https://www.mzcr.cz/tiskove-centrum-mz/vlada-schvalila-novelu-zakona-o-lecivech-ktera-zasadne-pomuze-odolnosti-ceskeho-lekoveho-trhu
State Institute for Drug Control. “Nedostupnost léčiv v ČR.” [Unavailability of medicines in the Czech Republic.] https://www.sukl.cz/nedostupnost-leciv-v-cr
Zdravotnický deník. Press news, 25 May 2023. “Novela zákona o léčivech bude během dvou týdnů na vládě, Hospodářská komora by chtěla stažení.” [The amendment to the Medicines Act will be presented to the government in two weeks, the Chamber of Commerce demands a withdrawal.] https://www.zdravotnickydenik.cz/2023/05/novela-zakona-o-lecivech-bude-behem-dvou-tydnu-na-vlade-hospodarska-komora-by-chtela-stazeni
The system has become more transparent for patients, and, since January 2022, changes to the reimbursement of innovative medicines and orphan drugs have been implemented by an amendment to the Health Insurance Act to improve their availability for Czech patients. Orphan drugs’ prices and reimbursement are now set in an automatic administrative procedure, and patients no longer have to rely on individual appeals to HIFs. Temporary reimbursement periods (for the highly innovative medical products) have been extended from two to three years for the first temporary reimbursement and from one to two years for the second temporary reimbursement. However, the marketing authorisation holder is obliged to reimburse HIFs for costs exceeding those indicated in the budget impact analysis that served as the basis for the SÚKL decision and there is a follow-up treatment obligation in case the product is not accepted for permanent reimbursement after the five-year period. Furthermore, new procedures for setting reimbursements overall were established for orphan drugs, involving medical societies and patient organisations.
A special advisory body at MZČR also now exists to make recommendations regarding orphan drugs. Importantly, patient representatives also sit on the advisory body, giving them a voice and direct access to important information. As such, the reimbursement level for orphan drugs is determined by the advisory body that newly contains more stakeholders. HIFs can also initiate reimbursement setting procedures; in these cases, market authorisation holders are not obliged to pay back costs exceeding the budget impact analysis that SÚKL based its issuance of the temporary reimbursement decision on. Expanded stakeholder involvement has also led to the establishment of a legal definition for patient organisations and a list of those recognised. The new process of reimbursement of orphan drugs should take also into account the quality of the patient's life and the impact on family members. This has been a significant step for patient involvement in the governance of the Czech health system, which is still very limited. In general, there are no patient boards in hospitals that would have to be consulted by management, nor are there patient representatives on VZP’s supervisory board.
Authors
References
2.7.5. Regulation of medical devices and aids
In line with Regulation (EU) 2017/745 on MDA, which came into effect in May 2021, the process of entering the Czech market has changed substantially. The EU regulation, plus a new national law (Act no. 89/2021 Coll.), replaced the two regulations related to the previous European Directives (90/385/EEC and 93/42/EEC). SÚKL is the regulatory body for MDA, with MZČR handling all related appeals. SÚKL began managing Czechia’s MDA Registry in 2015; its task portfolio was broadened in 2021 to also include administrative procedures of new applications to the registry, which is a necessary condition for a product to enter the market. The law allows for two types of temporary exemptions for a non-marketed product to be used: (1) for a specific patient based on MZČR approval; and (2) for a general exemption based on SÚKL approval for public health protection or for patient safety and health reasons.
Coverage of MDA is defined at the national level and adjustments to SHI reimbursements came into effect in 2019 with an amendment to the Health Insurance Act (1997). The amendment’s annex specifies the categorization of MDA for reimbursement (including prescription, indication, volume and reimbursement limits). SHI reimbursement levels can now only be changed through amendment to the law or temporarily when interchangeable MDA appear. In such cases, SÚKL defines the reference group of interchangeable MDA in an administrative procedure and organizes a bidding procedure. Alternatively, the level of reimbursement can be defined by an agreement on the highest price between a producer (distributor) and at least one HIF. For marketed MDA, SÚKL assigns an MDA to a particular reimbursement category based on the producer’s or distributor’s application, and administrative procedure is only initiated if SÚKL finds the application to be faulty and there is a risk of its rejection. For MDA needing a new categorization group, exemptions from the 2018 amendment exist in cases where risk-sharing contracts are signed with all HIFs or via MZČR approval.
