-
22 July 2024 | Country Update
Political interference in the Slovak health system
2.2. Organization
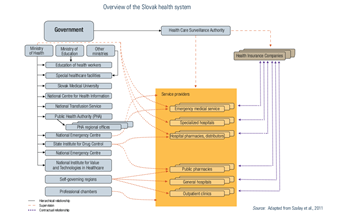
The organizational framework of the current health system was legislatively defined in 2004. Health policy results from the interplay between MZ SR (legislator), HICs (purchasers), providers, professional organizations and the ÚDZS as supervisor (see Fig2.1). While patient organizations’ influence is not legally codified, their comments on draft legislation are often incorporated.
Fig2.1
As explained in a previous policy analysis (https://eurohealthobservatory.who.int/monitors/health-systems-monitor/analyses/hspm/slovakia-2016/slovakia-s-health-care-surveillance-authority-s-lacking-institutional-stability-and-independence), the Health Care Surveillance Authority (Úrad pre dohľad nad zdravotnou starostlivosťou in Slovak) plays an important regulatory role in the Slovak health system and is responsible for supervising health insurance, purchasing and healthcare markets. As with previous governments (no chair of the Health Care Surveillance Authority has served a full term since its establishment in 2004), the current government led by Prime Minister Robert Fico with Minister of Heath Zuzana Dolinková have politicized the role of the Authority’s chair. In February 2024, they used legislative amendments to §22 of Act 581/2004 to remove the then-incumbent Renáta Blahová.1, 2
More recently, the Fico government has adopted legislation to take effect on 1 August 2024 to change the criteria of who can serve as director of the National Institute for Value and Technologies in Healthcare (NIHO or Národný inštitút pre hodnotu a technológie v zdravotníctve), which was established in 2022 and is responsible for Health Technology Assessment in Slovakia. The new criteria specify that only a doctor or pharmacist could serve as head of the HTA agency (leading to the dismissal of the current head, Michal Staňák); the legislation also enables the Minister of Heath to dismiss the agency’s director at any time and without cause.
Besides the political sphere pushing itself into the decision-making levels of these two seemingly independent organizations within the health system, the current government has also used their existing authority to make the following changes in healthcare institutions and providers around the country since coming into office in October 2023 (the dates refer to when the officials were dismissed or replaced):
- Tomáš Janík, director of Faculty Hospital Trenčín, 14 November 2023.3
- Vladislav Šrojta, director of Faculty Hospital Trnava, 30 November 2023.4
- Pavol Bartošík, general director of Central Slovak Institute of Heart and Vascular Diseases (Stredoslovenský ústav srdcových a cievnych chorôb), 5 December 2023.5
- Ľubomír Šarník, director of Faculty Hospital Prešov, 6 December 2023.6
- Jozef Tekáč, director of Faculty Hospital Poprad, December 2023.7
- Eduard Dorčík, director of the hospital in Žilina, December 2023.8
- Peter Potůček, director of State Institute for Drug Control (Štátny ústav pre kontrolu liečiv), 31 December 2023.9
- Ľubica Hlinková, general director of VšZP (Všeobecná zdravotná poisťovňa), the state-owned Health Insurance Company, 10 January 2024.10
- Peter Lukáč, director of National Centre for Health Information (Národné centrum zdravotníckych informácií), 10 January 2024.11
- Ivan Kocan, director of University Hospital Martin, 10 January 2024.12
- Július Pavčo, director of Emergency Medical Service Operations Centre (Operačné stredisko záchrannej zdravotnej služby), 31 January 2024.13
- Renáta Blahová, Chair of Health Care Surveillance Authority, 6 February 2024.14
- Michal Fajin, director of Faculty Hospital Nitra, 14 February 2024.15
- Matej Mišík, chief of the Institute for Healthcare Analyses (Inštitút zdravotných analýz) at the Ministry of Health was revoked on 20.6.2024 without any reason. The Institute focuses on: epidemiological studies and data analysis, sector analysis and health policies, implementation of optimization of the hospital network and the development of the DRG reimbursement mechanism.16
- Michal Staňák, director of NIHO, according to legislation set to take effect on 1 August 2024, enabling the Minister of Health to dismiss the NIHO at any time and without giving a reason. The Ministry of Health has already published the announcement for the selection procedure for the position of the new director.17
Authors
References
1. https://spectator.sme.sk/c/23346445/slovak-health-minister-direct-power-independent-body.html
5. https://www.health.gov.sk/Clanok?mz-suscch-vedenie-nove
6. https://domov.sme.sk/c/23281567/fajin-nemocnica-nitra-dolinkova-pellegrini-hlas-rozhovor.html
7. https://domov.sme.sk/c/23272033/zuzana-dolinkova-zdravotnictvo-nemocnice-cistky.html
8. https://dennikn.sk/minuta/3744432
11. https://zive.aktuality.sk/clanok/SdYXsqi/odvolali-riaditela-nczi-kto-bude-na-cele
13. https://www.health.gov.sk/Clanok?operacne-zachranka-riaditel
14. https://www.tyzden.sk/zdravotnictvo/106251/vlada-odvolala-sefku-udzs-na-jej-miesto-zasadne-palkovic
15. https://domov.sme.sk/c/23281567/fajin-nemocnica-nitra-dolinkova-pellegrini-hlas-rozhovor.html
2.2.1. The state and its agencies
Parliament (National Council)
The Slovak Parliament has legislative and oversight powers and may carry out parliamentary inspections. The five members of the ÚDZS Supervisory Board are elected by parliament based on the government’s proposal.
Government
The government approves the budgets of HICs, adopts legislative measures (defining cost-sharing such as user fees and setting co-payments, and setting reimbursements for dental and medical emergency care) and appoints the ÚDZS Chair, based on the Minister of Health’s proposal. The government also names the seven ÚDZS Board of Directors members based on MZ SR’s proposals.
Ministry of Health (MZ SR)
MZ SR’s responsibilities include developing health policy and drafting legislation, regulating provision, issuing diagnostic and therapeutic guidelines, managing national health programmes, partial management of health education, determining the scope of the basic benefits package, defining health indicators and setting minimum quality criteria. MZ SR also issues permits for treatments with natural healing/mineral waters and the operation of natural healing spas.
MZ SR prepares budget allocations for health services following consultations with HICs and providers. For inpatient care, MZ SR issues diagnosis-related group (DRG) weights and since 2022 has actively participated in concluding managed entry agreements with pharmaceutical product registration holders (see Section 2.7.4).
MZ SR owns almost all university and faculty hospitals,[2] sanatoria, the largest HIC (General Health Insurance Company, Všeobecná zdravotná poisťovňa, VšZP) and five highly specialized hospitals. MZ SR is also a founder or co-founder of 21 non-profit organizations, including specialized centres or smaller regional hospitals. The Minister of Health appoints the directors of all state-owned providers. Finally, MZ SR also publishes data on the scope, structure and deadlines for submitting clinical and economic data from providers, as well as price regulation.
Other ministries
The management and supervision of health education and the curriculum are shared between MZ SR and the Ministry of Education, the latter being responsible for the financing. MZ SR coordinates health research in universities and the Academy of Sciences. The Ministry of Finance also monitors expenditure and reports on cost-saving and value-oriented measures that can be incorporated into public administration budgets, while HICs have to report to the Ministry of Finance.
The organization and funding of social care, including sick leave, is the responsibility of the Ministry of Labour, Social Affairs and Family. The social system and health systems evolved separately, leading to different organizations and sources of funding, even though many services they provide are practically identical. This complicates the sustainable provision of long-term care (LTC) (see Section 5.8).
The Ministries of the Interior, Justice, Defence and Transport have established health care facilities, notably the Military Hospital in Ružomberok and St Michal Hospital, and play a marginal role in health care provision.
Health Care Surveillance Authority (ÚDZS)
In 2004, to prevent potential conflicts of interests, the supervisory role of MZ SR was transferred to the newly established ÚDZS, regarding (1) health insurance, (2) provision and delivery of services and (3) purchasing. ÚDZS can sanction, including banning providers from the market. Moreover, it administers the risk-adjustment mechanism between HICs and administers patients’ complaints regarding inadequate provision. ÚDZS also acts as a liaison for cross-border provision, and its annual report to the government describes activities and HICs’ performance. Between 2010 and 2020 ÚDZS was also responsible for the implementation of Slovakia’s DRG system.
ÚDZS Chairs are elected for five-year terms. When introduced in 2004, the Chair’s irrevocability from the office was adopted to guarantee their independence and they could only be removed after committing crimes or in case of death. This provision has been repealed and reintroduced twice; currently, the ÚDZS Chair’s mandate is revocable from office by the Government at the suggestion of MZ SR. Since ÚDZS’ establishment, not one of the seven Chairs has served a full term: their mandates were either revoked (three times) or they resigned on their own (four times). ÚDZS could previously intervene to order a recovery plan in the case of unfavourable management of a HIC (i.e., if the HIC’s losses were such that its equity fell below the minimum value of share capital of €16 600 000), but in 2022 this was legislatively altered and ÚDZS can now only suggest this.
Public Health Authority of the Slovak Republic (Úrad verejného zdravotníctva Slovenskej republiky, ÚVZ SR)
ÚVZ SR’s main objectives are to protect, support and promote public health in Slovakia (see Section 5.1). It is headed by the Chief Public Health Officer. ÚVZ SR acts as the supervisory body over 36 regional public health authorities, including over laboratories and national reference centres. ÚVZ SR oversees the direction and priorities of national policy in the area of public health and also develops vaccination schedules, directly controls radiation protection and issues permits for selling cosmetics. Through the regional public health authorities, ÚVZ SR carries out epidemiological surveillance, assesses environmental factors’ impact on health, and monitors the quality of drinking and bathing water. ÚVZ SR can impose sanctions (for example, for avoiding mandatory vaccinations). Further competences include promoting food safety and hygiene, national and international cooperation, radiation protection, and preventive and occupational medicine.
ÚVZ SR’s importance was demonstrated during the COVID-19 pandemic, regularly issuing measures to protect public health (quarantine, limiting gatherings, banning visits to inpatient facilities, etc.).
State Institute for Drug Control (Štátny ústav pre kontrolu liečiv, ŠÚKL)
ŠÚKL is responsible for surveillance of medicines and medical devices. It approves clinical trials, grants marketing authorizations, assesses pharmacies and maintains a pharmacopoeia. ŠÚKL also assesses reports on adverse drug effects (pharmacovigilance) and medical device failures, and can withdraw or suspend medicines or medical devices, though it is not involved in reimbursement decisions.
ŠÚKL regulates re-exports that can cause limited availability of some medicines. To limit this practice, it can impose sanctions for those who illegally re-export medicines. To export medicines from the positive list of categorized medicines (i.e., the reimbursement list, see Section 2.7.4), a company must notify ŠÚKL electronically within seven days of the export. Notification regarding non-categorized medicines is longer (quarterly).
National Institute for Value and Technologies in Healthcare (Národný inštitút pre hodnotu a technológie v zdravotníctve, NIHO)
NIHO was established in 2022 and is responsible for health technology assessment (HTA) in Slovakia. It publishes evaluations and analyses based on established European methodologies on evidence-based medicine. For pharmaceuticals, its key role is to prepare evaluations of medicines with a potential impact on health insurance for the purposes of categorization and reimbursements.
The director is appointed by the Minister of Health for five years and the appointment can be revoked without specific reason; the Supervisory Board has four members serving three years (Slov-Lex, 2024a).
Emergency Medical Service Operations Centre (Operačné stredisko záchrannej zdravotnej služby, OS ZZS)
OS ZZS oversees emergency medical services. It has one central office and eight regional centres that control and coordinate integrated rescue systems, together with key stakeholders. OS ZZS processes all emergency calls and coordinates with emergency ambulance crews; manages, coordinates and evaluates services to ensure smooth operation; provides employee training; and organizes first aid and first aid instructor courses.
National Centre for Health Information (Národné centrum zdravotníckych informácií, NCZI)
MZ SR established the NCZI to develop eHealth and standardize health information systems and the collection, processing and provision of health statistics. NCZI operates the national health registers as well as providing library and information services for medical research and health. NCZI also operates the National Health Information System (NHIS). Although implementation of NHIS has been delayed, only a few functionalities are used regularly (see Section 4.1.3). Future ambitions are to offer electronic services for prescriptions, examinations, vaccinations and laboratory services, health records and coordination of appointments.
National Transfusion Service (Národná transfúzna služba, NTS)
NTS was established in 2004 by MZ SR to carry out tasks related to transfusions and blood donations, maintaining high quality and safety.
National Transplant Organization (Národná transplantačná organizácia, NTO)
NTO was established by MZ SR in 2013. Tasks include national donation coordination of organs, tissues and cells, and running the national transplant register, which includes waiting lists for transplants, registering donors and keeping records.
Slovak Medical University (Slovenská zdravotnícka univerzita, SZU)
SZU operates under MZ SR and has four faculties:
- Faculty of Nursing and Professional Health Studies
- Faculty of Medicine
- Faculty of Public Health
- Faculty of Health.
SZU instructs health care professionals across all three levels of higher education, specialized studies and continuous medical education in all relevant fields. Additionally, SZU researches medical and pharmaceutical sciences.
Healthy Regions (Zdravé regióny, ZR)
ZR carries out initiatives aimed at systematically enhancing conditions of marginalized Roma communities to attain improved population health results. Main activities are targeted around national projects financed by the European Structural and Investment Funds (Zdravé Regióny, 2022).
ZR also aims to reach the Roma community by promoting access to health care, including prevention and health education, as well as reducing health gaps between Roma and the general population. The programme is currently being carried out in 245 locations, mainly in central and eastern Slovakia.
- 2. Faculty hospitals have a contract with a university and conduct training for health care professionals in non-medical fields, while university hospitals have a contract with a university and conduct training for medical doctors. ↰
2.2.2. HICs
HICs play a key role by collecting, pooling and redistributing resources and purchasing services. With the exemption of DRGs, the rescue emergency system, drugs and medical devices reimbursements, HICs are allowed to develop their own payment mechanisms and arrange pricing policy with contracted providers. Purchasing is based on selective contracting and the contractual relations between HICs and providers are supervised by ÚDZS (see Section 3.3.4).
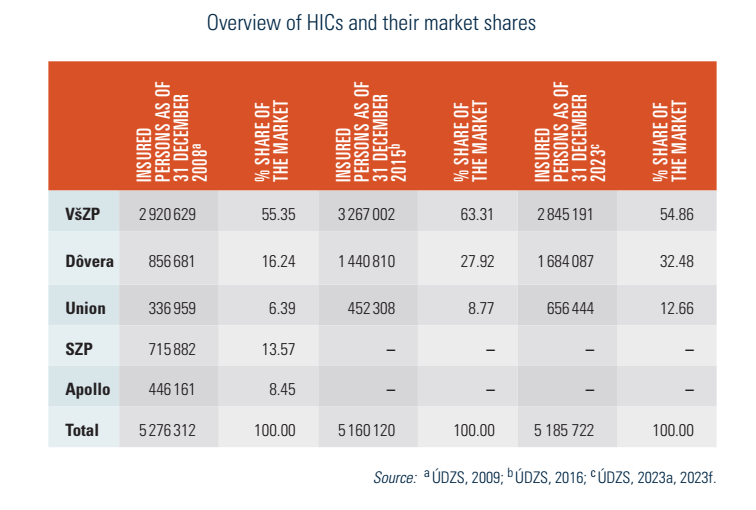
HICs are joint-stock companies and are obliged to meet solvency criteria. This guarantees scheduled payments within 30 days after the issuing of a provider’s invoice. Ownership regulation allows both the state and the private sector to be shareholders of HICs. Although there were seven HICs in 2006, a wave of mergers led to increased consolidation in the market and there are three as of 2024: the state-owned VšZP and two privately owned ones (Dôvera and Union) (see Table2.1).
Table2.1
2.2.3. SGRs
SGRs’ responsibilities include issuing permits for the operation of health care facilities (hospitals, outpatient facilities), approving office hours, appointing ethical committees, issuing outpatient emergency service schedules, approving outpatient biomedical research and archiving medical paper records after a provider’s closure or retirement. MZ SR reviews appeals against decisions made by the SGRs. The SGRs also assist in improving the provider networks to guarantee access.
SGRs monitor the provision of care and can sanction providers for neglecting their duties, including financial penalties and – after a recommendation from ÚDZS – permit revocations. SGRs own some facilities and can independently manage them. Since gaining this competence in 2003 (see Section 2.3) most of those facilities were leased to private providers.
During the COVID-19 pandemic, SGRs played a crucial role in mass testing in cooperation with district authorities (i.e., logistics and organization). Moreover, SGRs also organized vaccinations in large-capacity centres and the mobile vaccination service.
SGRs have also played a crucial role in meeting the needs of refugees from Ukraine (see Box2.1).
Box2.1
2.2.4. Non-state actors
Professional organizations
Organizations of providers and professional chambers promote and advocate the interests of their members. They comment on draft legislation and represent their members in contract negotiations with HICs. They maintain workforce registers and offer continuous education. Chambers also grant licences and impose sanctions. Since 2005 chamber membership has been voluntary (issuing licences and maintaining registers of health professionals are distinct from membership in the chamber), though the oldest chambers have managed to retain members and thus influence. These include:
- Slovak Medical Chamber (Slovenská lekárska komora, SLK)
- Slovak Chamber of Dental Physicians (Slovenská komora zubných lekárov)
- Slovak Pharmaceutical Chamber (Slovenská lekárnická komora)
- Slovak Chamber of Nurses and Midwives (Slovenská komora sestier a pôrodných asistentiek)
- Slovak Chamber of Medical and Technical Workers (Slovenská komora medicínsko-technických pracovníkov)
- Slovak Chamber of Physiotherapists (Slovenská komora fyzioterapeutov)
- Slovak Chamber of Dental Technicians (Slovenská komora zubných technikov)
- Slovak Chamber of Orthopaedic Technicians (Slovenská komora ortopedických technikov)
- Slovak Chamber of Other Healthcare Workers (Slovenská komora iných zdravotníckych pracovníkov)
- Slovak Chamber of Psychologists (Slovenská komora psychológov)
- Slovak Chamber of Emergency Medical Technicians (Slovenská komora zdravotníckych záchranárov).
The most significant organizations of providers are the Association of Hospitals of Slovakia (Asociácia nemocníc Slovenska) and the Association of Private Physicians of Slovakia (Asociácia súkromných lekárov).
The Slovak Medical Association (Slovenska lekárska spoločnosť) comprises medical and pharmaceutical groups and regional associations of physicians and pharmacists. It focuses on technical and ethical issues, as well as the dissemination of scientific knowledge. Professional societies within the Slovak Medical Association delegate their professionals to serve on committees (such as the Reimbursement Committee for Medicinal Products and the Catalogue Committee for medical procedures at MZ SR).
Private sector
Common interests are represented by umbrella organizations listed below, particularly regarding pharmaceuticals and devices:
- Association of Drug and Health Device Suppliers (Asociácia dodávateľov liekov a zdravotníckych pomôcok)
- Slovak Association of Medical Device Suppliers (Slovenská asociácia dodávateľov zdravotníckych pomôcok)
- Association of Innovative Pharmaceutical Industries (Asociácia inovatívneho farmaceutického priemyslu)
- Association for Generic and Biosimilar Drugs (Asociácia pre generické a biosimilárne lieky)
- Association of Health Insurance Companies Slovakia (Asociácia zdravotných poisťovní Slovenskej republiky; represents the three HICs).
Trade unions
The largest trade union (18 000 members) is the Association of Health and Social Trade Unions (Slovenský odborový zväz zdravotníctva a sociálnych služieb). It negotiates collective contracts with the employers’ representatives. The Trade Union of Physicians (Lekárske odborové druženie, LOZ) is smaller and mainly advocates financial interests. It played an important role in the 2022 mass protests of hospital-employed physicians over pay and work conditions, achieving significant benefits (see Section 3.7.2) (LOZ, 2022).
Patient/consumer groups
These are mostly determined by dedicated individuals and available financial resources and are represented by various umbrella organizations. Their work is often multidisciplinary, given the division of competences between health and social care (i.e., those with disabilities lobby the Ministry of Labour, Social Affairs and Family).
In the health system, patient organizations representing people with chronic conditions include:
- Slovak Diabetes Society (Slovenská diabetologická spoločnosť)
- Slovak Association of Multiple Sclerosis (Slovenský zväz sclerosis multiplex)
- Slovak Crohn Club
- League against Rheumatism in Slovakia (Liga proti reumatizmu na Slovensku)
- Club of Cystic Fibrosis (Klub cystickej fibrózy)
- Community for Help to People with Autism (Spoločnosť na pomoc osobám s autizmom)
- Slovakian Down Syndrome Association (Spoločnosť Downovho syndrómu na Slovensku).
Numerous projects for oncology patients, their relatives and the public are undertaken by the Slovak League against Cancer (Liga proti rakovine Slovenskej republiky), and the civic associations Nie rakovine and the Amazonky. Psychiatrists, psychotherapists and patient organizations cooperate within the League for Mental Health (Liga za duševné zdravie) to advocate for mental health.
The Association for the Protection of Patients’ Rights (Asociácia na ochranu práv pacientov SR, AOPP) brings together roughly 50 patient organizations to proactively participate in the legislative and categorization processes, and to set standards for diagnosis and treatment, pricing, and patient access to treatment (European Patients Forum, n.d.).