-
25 August 2025 | Country Update
Reimbursement Act amended to strengthen Health Technology Assessment -
10 October 2023 | Country Update
New law on quality in healthcare and patient safety published -
10 October 2023 | Country Update
New Law on Certain Health Professions -
28 September 2023 | Policy Analysis
Amendment to the Pharmacy for Pharmacists Act (2017) -
28 December 2021 | Country Update
Publication of the new strategy for the health sector “Healthy Future. A Strategic Framework for the Development of the Health Care System for 2021–2027, with a perspective until 2030” -
12 December 2021 | Country Update
Introduction of the National Health Programme 2021–2025 -
01 May 2020 | Country Update
Introduction of the National Cancer Strategy 2020–2030
2.4. Regulation and planning
The new strategy for the health sector “Healthy Future. A Strategic Framework for the Development of the Health Care System for 2021–2027, with a perspective until 2030” was adopted by the Council of Ministers on 27 December 2021.
The strategy defines actions aimed at improving the Polish health system in six main areas:
- Development of disease prevention and health promotion;
- Improving quality, user-friendliness and efficiency of health services through standardization and reorganization of care;
- Improving access to and effectiveness of health services through the development and modernization of health sector infrastructure;
- Supporting the development of human resources for health;
- Development of digital health services in the public health system;
- Development and increasing the use of modern technologies in the health sector.
The strategy is supplemented by two deinstitutionalization strategies: for older people and for psychiatric care.
The National Health Programme is the key strategic document in the area of public health in Poland. It is published periodically with the current edition in place between 2021 and 2025. Its primary objective is to increase the number of healthy years lived and reduce social inequalities in health by addressing the aftermath of the COVID-19 pandemic. It has the following operational goals:
- Prevention of overweight and obesity;
- Addiction prevention;
- Promotion of mental health;
- Environmental health and infectious diseases;
- Demographic challenges.
Some operational gaols are subdivided into specific sub-areas and each operational goal comprises specific tasks and indicates entities responsible for their realisation. The executive regulation of the Council of Minister that introduced the Programme specifies how the Programme will be financed, monitored and evaluated. The Minister of Health is responsible for supervising the Programme, but its implementation involves coordination across various departments of the government.
References
Rozporządzenie Rady Ministrów z dnia 30 marca 2021 r. w sprawie Narodowego Programu Zdrowia na lata 2021-2025 Dz.U. 2021 poz. 642: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20210000642/O/D20210642.pdf
The National Cancer Strategy 2020–2030 came into force in April 2020,
replacing the National Programme for Cancer Diseases Control for
2016–2024. The Strategy represents the first national cancer plan that
comprehensively covers all aspects of cancer care in Poland. It is also
aligned with Europe’s Beating Cancer Plan.
The goal of the Strategy is to increase the five-year survival rates after cancer treatment, improve early detection of cancers and increase the quality of life during and after treatment. The Strategy focuses on investments in five areas:
- human resources;
- education about healthy lifestyles (including by involving primary care doctors and occupational medicine doctors in primary cancer prevention);
- patients (including by increasing access to cancer screening);
- science and innovation; and
- oncology care system (including by supporting the introduction of the National Oncological Network, which has been piloted since 2019 – see the Policy Analysis on this topic).
Authors
2.4.1. Planning
Planning in the health system is the responsibility of the central government administration, particularly of the Minister of Health and the voivodes. The Minister of Health and the voivodes are supported in this function by a variety of institutions, many of them created fairly recently. Key planning documents include:
- The Long-term National Development Strategy: Poland 2030. Third wave of modernity (Długookresowa Strategia Rozwoju Kraju: Polska 2030. Trzecia fala nowoczesności), complemented by the Strategy for Responsible Development until 2020 (with perspective until 2030) (Strategia na rzecz Odpowiedzialnego Rozwoju do roku 2020 (z perspektywą do 2030 r.)),[3] which is the key strategic document in the area of national economic policy. These documents, developed by the Ministry of Regional Development, define the vision for the country’s development in the medium- and long-term.
- The National Strategic Framework. Policy paper for health protection for 2014–2020, which sets out priorities for the health system in connection with the planned measures that are to be financed with the support from EU Structural Funds allocated for the years 2014–2020 (for more information on the strategic objectives stated in this document, see section 7.1).
- The National Health Programmes (NPZs) are the key medium-term national health strategy documents in the area of public health. The current Programme was formulated for the 2016–2020 period.
- Annual Health needs maps, introduced in 2015, are the key medium- to long-term health policy planning document.
Health needs maps were introduced as a decision-support tool, at both operational (i.e. at the level of health care providers) and strategic (local and national) levels. Their purpose is to identify current and future health needs and demand for health care services (Więckowska, 2017). This may be used to improve contracting (though contracting will also largely be determined by the available resources and existing health system infrastructure) (see sections 3.3.3. and 3.3.4) and to determine priorities for the health policy. Regional health needs maps are developed by the voivode (based on drafts prepared by the NIZP-PZH; see Table2.1) in consultation with the Voivodeship Health Needs Councils composed of voivodeship consultants and representatives of institutions such as the NFZ, Voivodeship Statistical Offices, Voivodeship Marshals, medical colleges, employers’ organizations, and so on. Regional health needs maps are approved by the Minister of Health. The first maps were published in December 2015 and focused on oncology and cardiology treatment. Since then maps have been issued in various areas of hospital care, outpatient specialist care and primary care, and further maps are under preparation.
Table2.1
Planning of health services provision is largely determined by the AOTMiT (see Table2.1) and the NFZ. AOTMiT, with the advice from the national consultants, makes recommendations on the guaranteed benefits and their tariffs to the Minister of Health, who approves them. The president of the NFZ prepares the annual financial plan for the Fund. This plan must be approved by the Minister of Health in agreement with the Minister of Finance. According to this plan, NFZ’s voivodeship branches contract services with health care providers. If demand for services exceeds what had been budgeted for, non-urgent services are rationed via waiting lists and are accounted for in the next year’s budget. The voivodes are responsible for planning the provision of emergency medical services. This is done via the Voivodeship Emergency Rescue System Operation Plans (which are approved by the Minister of Health). Plans for managing emergencies and providing assistance to victims of major disasters are also developed at the regional level and are coordinated centrally.
The Minister of Health and the voivodes, with the aid of national and voivodeship consultants, are responsible for planning of medical training based on the assessment of the population’s health needs and training capacities. This involves determining the admission limits of particular medical colleges (only for physicians and dentists; see section 4.2.1) and the number of residency positions to be allocated for each voivodeship in each medical specialization. The inclusion in the hospital network (see section 3.3.4) as well as planning on the part of the NFZ (which determines how many services will be contracted and from how many service providers outside the hospital network) influence the planning of medical training in the voivodeships.
Box2.2 describes evaluation of priority-setting and planning.
Box2.2
- 3. This Strategy updated the Medium-Term National Development Strategy and Nine Horizontal Strategies (until 2020). For more information see: https://www.miir.gov.pl/strony/strategia-na-rzecz-odpowiedzialnego-rozwoju/informacje-o-strategii/ ↰
2.4.2. Regulation
The health sector is extensively regulated. Regulations regarding standard setting and implementation mainly concern health care financing, training of medical personnel, conditions in which health services are delivered to patients, operation of service providers, assuring availability of health care services and medicines (including the level of cost-sharing) and assuring observance of patient rights (Table2.2). Some of the regulations, such as those on cross-border care and marketing authorization for medicines, stem from Poland’s membership in the EU and ensure conformity with the relevant EU directives and other regulations.
Table2.2
In the process of decentralization, some of the regulatory functions have been transferred to the territorial self-governments. Local authorities may adopt resolutions on various matters which have indirect (e.g. resolutions banning coal use for heating) or direct (resolutions concerning financing of in vitro fertilization (IVF)) relevance for health. Local authorities also plan and implement local health policy programmes and perform regulatory functions related to their ownership of public health care providers.
Monitoring and evaluation functions are institutionally not sufficiently developed or coordinated. They are carried out by various supervisory bodies, among which the Chief Sanitary Inspectorate has the strongest position. Deficiencies in the area of monitoring are particularly evident in relation to private health care entities that do not receive public financing (i.e. entities that are not contracted by the NFZ). For example, such entities often do not monitor and evaluate the services they provide, which is required from all providers contracted by the NFZ (although the largest private providers do so voluntarily for reputational reasons).
2.4.3. Regulation and governance of third-party payers
The NFZ is the sole payer in the mandatory health insurance system. It operates on a non-profit basis. Its annual financial plan must be approved by the Minister of Health and the Minister of Finance. The NFZ must assure transparency of public financing by granting public access to key information about its annual financial plan and its implementation as well as on the contracts concluded with health care providers. The legislation prohibits the NFZ from any involvement in the provision of health care services thus creating a strict separation between public financing and the internal market of health care provision. The 16 voivodeship branches of the NFZ are subordinate to the NFZ’s Central Office (deconcentration) and are responsible for contracting services.
Lists of health services financed from public sources (NFZ or other), including levels of patient cost-sharing, price limits and conditions in which these services should be rendered (such as requirements on medical personnel and medical equipment), were specified in 2009 by way of 13 executive regulations of the Minister of Health (see section 3.3.1). Prior to that, the benefits basket was defined through a variety of legal acts, but was nevertheless very explicit. Drug reimbursement (list of reimbursed drugs and the level of reimbursement) is regulated by the 2011 Act on the Reimbursement of Pharmaceuticals, Foodstuffs for Special Nutritional Use and Medical Devices (“Act on Reimbursement”). Until recently, contracting of services by the NFZ was done via competitive tenders (see section 3.3.4) and was based on the Plans for Purchase of Benefits developed by the NFZ. Since the introduction of the hospital network in October 2017, qualifying hospitals have been automatically granted a contract with their local NFZ branch for a period of four years. Traditional contracting only applies to hospitals not included in the network, to certain non-hospital services, and to certain hospital services that are not covered within the hospital network, e.g. one-day orthopaedics surgery. The NFZ is not permitted to have debts. If demand for services is higher than the number of contracted services, services are rationed via waiting lists.
Regulation of third-party health care payer institutions is primarily focused on the public system and on the NFZ as its core. Existing legislation (the 2004 Act on Health Care Services Financed from Public Sources) does not allow for the establishment of private complementary health insurance that covers benefits that are excluded from the statutory benefits baskets or only partly covered. Private supplementary voluntary health insurance (VHI) exists, mainly in the form of medical prepaid subscriptions (a quasi-insurance product) offered by private health care provider companies that also provide medical services themselves (treatment can also be received within the network of providers belonging to the company offering subscriptions or from cooperating providers) (Sobczak, 2016; see also section 3.5). This system, also termed “quasi-insurance”, follows the United States of America’s example of Health Maintenance Organizations (HMOs), where financing is integrated with health services provision. However, the majority of medical subscriptions’ providers have contracts for provision of services with health insurance companies (Wiedziuk, 2018). Subscriptions are not legally recognized as an insurance product and do not fall under the 2003 Act on Insurance Activity; they are therefore not part of the financial sector and not subject to supervision in the same way as registered health insurers (which may be considered to constitute unfair competition). Medical subscriptions are rooted in employers’ legal obligation to provide employees with occupational health services. However, they often also cover other medical services for employees and their families, giving their beneficiaries access to health services in the event of health problems (akin to an insurance product).
Private supplementary VHI is also offered by registered insurance institutions (often together with other insurance products, such as life insurance) and may allow for the reimbursement of treatment received from any chosen health care provider (including ones located abroad) or be based on contracts with selected providers. It is prohibited for an insurance company to provide any other activity that is not directly related to insurance such as provision of health care services. In most cases private health insurance benefits are provided in kind, on the basis of contracts with health care providers (Osak, 2016). Commercial health insurance falls within the 2015 Act on Insurance and Reinsurance Activity and thus under the general supervision by the Minister of Finance and the Polish Financial Supervision Authority.
2.4.4. Regulation and governance of provision
All entities that provide health services in Poland are included in a central register that holds information on the types of services they provide (Rejestr Podmiotów Wykonujących Działalność Leczniczą, RPWDL). Supervision of health care providers is exercised by the Minister of Health (overall activity), the voivodes and professional chambers (registration process), within the system of the Chief Sanitary Inspectorate (covering sanitary requirements for health care facilities), and by the NFZ (supervision of contracts). See Fig2.2 for the types of health care providers. Table2.3 provides overview of the regulation of providers.
| Fig2.2 | Table2.3 |
 |  |
The legal status of entities providing health services was specified in the 2011 Act on Therapeutic Activity. The majority of public hospitals operate as SPZOZs (see Box2.3).
Box2.3
Founders of health care providers (in case of public providers these are the Minister of Health and the territorial self-governments) must ensure that services are accessible and of adequate quality. Health care facilities must meet standards regarding the premises (minimum room standards), medical equipment and medical personnel (minimum standards for the number and qualification of personnel) – these requirements must be met by any provider rendering services included in the statutory benefits basket.
Accreditation was introduced in 1998 for hospitals and in 2016 for primary care providers. The principles and procedures concerning the process of granting accreditation are laid down in the 2008 Act on Accreditation in Health Care (originally laid down in the 1991 Act on Health Care Units). Accreditation of health care providers is voluntary. For hospitals, benefits of accreditation include a 10% reduction in the cost of insurance against adverse medical events, extra points in public tenders for contracts with the NFZ, and larger budgets (by 1–2%, depending on the number of accreditation points) for hospitals included in the hospital network. So far, no such benefits are available for primary care providers. Primary care providers that seek to obtain accreditation can receive support (e.g. training) in the accreditation process. Accreditation is granted by the Minister of Health, on the basis of recommendations from the Accreditation Council that follows an evaluation by the Centre for Quality Monitoring in Health Care (CMJ). Hospitals must obtain at least 75% of the maximal number of accreditation points. Accreditation is granted for the period of 3 years. The website of the Polish Accreditation Centre (Polskie Centrum Akredytacji, PCA) lists all accredited hospitals and PHC units. As of November 2018, 190 hospitals (less than 20% of the total number) and 42 primary care providers (less than 1% of the total number) were accredited (CMJ, 2018b).
Clinical pathways depend in most cases on the attending primary care physician or specialist. However, care coordination is progressively being introduced for various patient groups and conditions (see Table5.2).
Table5.2
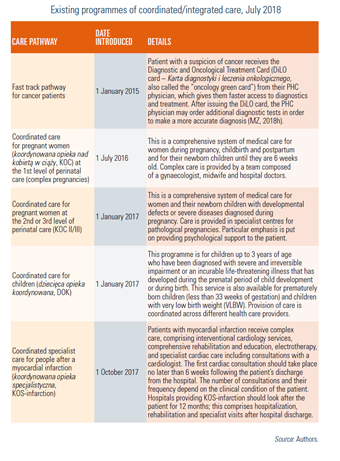
The Law of 16 June 2023 on quality in healthcare and patient safety was published on 24 August 2023. The key features include:
- Authorization: the Act stipulates that the authorization requirement applies only to hospitals. Applications for authorization will be accepted 6 months after the Quality Act comes into force. Authorization to provide state-financed services is granted to hospitals by the National Health Fund (NHF) for a period of 5 years.
- An internal quality and safety management system: the internal system, according to the Act, consists of rules, procedures, methods, and job descriptions to prevent adverse events. The obligation to implement such a system applies only to institutions providing services financed under a contract with the NHF.
- Accreditation: this confirms that the institution providing medical services meets the accreditation standards. According to the Act, accreditation is granted by the Minister of Health for a period of 4 years in the form of a certificate. According to the Act, the Minister of Health grants accreditation after receiving a recommendation from the Accreditation Council.
- Medical registers. The Act also provides for the reporting and sharing of data from medical registers. It allows public access to data from such registers in an anonymized form. Entities maintaining medical registers are obliged to make part of the data available in a format determined and disseminated by the Minister of Health and to prepare and publish reports based on the data from these registers at least once a year.
Authors
References
Law of 16 June 2023 on quality in healthcare and patient safety, JoL 2023. item.1692
Authors
References
2.4.5. Regulation of services and goods
Basic benefit package
The 2009 Act on the Amendment of the Act on Health Care Services Financed from Public Sources and the Act on Prices (“Act on Health Benefits Package”) introduced the basic package of statutory health care benefits. In accordance with this Act, the Minister of Health determines lists of statutory health care benefits via executive regulations, although the 2011 Act on Reimbursement provides some exceptions to this rule. These regulations cover PHC services; outpatient specialist care; inpatient care; mandatory vaccinations; therapeutic health programmes; and highly specialized benefits.
The Minister of Health decides on the inclusion (or exclusion) of a particular service in the guaranteed benefits basket, changes in the level of public financing and changes in the conditions in which the service is rendered. These decisions are informed by the AOTMiT (see the Health technology assessment section). Apart from the Minister of Health, the president of the NFZ and the national consultants as well as (via the national consultants) national scientific associations and NGOs involved in patient rights protection may initiate the procedure for including a particular service in the guaranteed benefits basket (or its removal).
Health technology assessment
The AOTMiT was established in 2005 as an advisory body to the Minister of Health. Its role has grown gradually and in 2015 the Agency became responsible for setting tariffs for health care services (see Table2.1). Before the Agency was created, there was no public entity in the Polish health care system whose main activity was the assessment of medical technologies financed from public sources. However, some activities related to HTA were undertaken by the NFZ and by the CMJ, which was established in 1994.
Table2.1
The Agency’s main area of activity is appraisal of medicines, although health care services and public health policy programmes are also appraised. In 2018, the Agency carried out 235 appraisals commissioned by the Ministry of Health (207 in 2017, 227 in 2016 and 219 in 2015) (AOTMiT, 2019). For medicines, the procedure is initiated by the Marketing Authorization Holder (MAH) who submits a standard application for the inclusion of the medicine in the list of publicly reimbursed medicines. In case of innovative products (without any equivalents on the reimbursement list), in case of a new clinical indication for a product that is already included in the reimbursement list, or if the MAH is asking for a higher price for a product that is already reimbursed, the MAH must additionally submit an HTA report. The full report consists of decision problem analysis,[4] clinical analysis, economic analysis and analysis of impact on the health care system (including a budget impact assessment) (AOTMiT, 2016). The report should be prepared following the HTA guidelines which have been issued and periodically updated by the HTA state agency in 2007, 2009 and 2016 (AOTMiT, 2016). The report is critically assessed by the AOTMiT staff and, independently, by the Transparency Council (see Table2.1). The president of the Agency submits their final recommendation to the Minister of Health, together with the results of these assessments. The recommendations are made public but they are partly censored in order to protect trade secrets or personal data. The extent of censoring used to be high, but transparency of the process improved since 2014 following changes in the interpretation of the existing legislation (Bochenek et al., 2016). Before the Minister of Health announces the final decision on the reimbursement, the applicant negotiates it with the Economic Commission, which convenes regularly at the Ministry of Health in Warsaw.
The AOTMiT has developed collaborations with its counterparts in other countries, including Health Technology Assessment International (HTAi), International Network of Agencies for Health Technology Assessment (INAHTA), Medicine Evaluation Committee (MEDEV), International Society for Pharmacoeconomics and Outcomes Research (ISPOR), and the European Network for Health Technology Assessment (EUnetHTA). Collaboration mainly covers methodological issues.
- 4. This includes an overview of the basic information pertaining to the assessed technology and the given health problem, its target population, achieved health outcomes and alternative technologies. ↰
Authors
2.4.6. Regulation and governance of pharmaceuticals
For information on pharmaceutical care, see section 5.6.
Regulation of pharmaceutical products
Market authorization
The regulatory body which is responsible for the issuance of marketing authorizations, i.e. registration of medicines in Poland (as well as their withdrawal) is the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products (Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, URPLWMiPB). The Office is subordinated to the Minister of Health, who appoints its president and supervises its operations. The Office can withdraw a previously granted market authorization in case of unexpected and severe or life-threatening adverse effects. Authorization may also be withdrawn in case of lack of declared therapeutic efficacy or when the risk of applying the product is incommensurate with its therapeutic effect.
The process of issuing marketing authorizations for medicines has been harmonized with EU regulations. The following procedures are used for registration of medicinal products in Poland: centralized procedure (performed at the level of the EC and the European Medicines Agency (EMA)), national procedure, decentralized procedure and mutual recognition procedure – all four compliant with EU standards. Medicinal products authorized in Poland are registered by the president of the URPLWMiPB. Market authorizations are valid for 5 years but can be extended (even indefinitely) on request of the responsible entity (i.e. a pharmaceutical company). The following products may be sold in Poland without a marketing authorization: magistral formula (medicinal product prepared in a pharmacy in accordance with a physician’s prescription for an individual patient), officinal formula (medicinal product prepared in a pharmacy in accordance with the prescriptions of a pharmacopoeia and intended to be supplied directly to the patients served by the pharmacy), radiopharmaceutical products, blood and plasma, pharmaceutical raw materials not intended for production of medicines, veterinary immunological products and tested medicinal products exclusively used for clinical or scientific trials.
Quality of medicines
The State Pharmaceutical Inspection (Państwowa Inspekcja Farmaceutyczna, PIF) supervises the quality of medicines on the Polish market (see Table2.1). This includes monitoring the conditions of the manufacture, import and distribution of medicinal products and active substances, as well as marketing of medicinal products (URPLWMiPB, 2018). Basic quality requirements and testing methods for medicinal products and their packaging and for pharmaceutical raw materials are specified in the Polish Pharmacopoeia, which is fully compatible with the European Pharmacopoeia.
Table2.1
Pharmacovigilance
The pharmacovigilance system was put in place in 1971 and, since 2013, not only representatives of medical professions, but also patients are able to report adverse reactions to medicinal products. Safety of medicines is supervised by the president of the URPLWMiPB. Entities that have obtained market authorization for medicinal products must continuously monitor their safety by keeping records of all adverse effects reported by physicians, pharmacists or patients undergoing treatment and to present yearly reports to the Pharmacovigilance Department at the URPLWMiPB.
Patent protection
Medicinal products in Poland have been able to be protected by patents since 1993. Since its EU accession in 2004, Poland has granted the same period of market exclusivity as in the EU, which is typically 20 years. Poland also agreed to introduce a supplementary patent protection (Supplementary Protection Certificate), which grants up to 5 years of additional patent protection (Kęska, Sławatyniec & Deloitte Legal, 2015). The research exemption (safe harbour exemption) is recognized in Poland, meaning that generic manufacturers are allowed to prepare generic drugs in advance of the patent expiration, without infringing the patent protection.
Advertising
Advertising of medicinal products is regulated by the 2001 Act on the Pharmaceutical Law. The Act bans public advertising of prescription medications and of medicinal products containing narcotic and psychotropic substances, drugs used exclusively in hospital treatment, prescription drugs and drugs not authorized for sale in the Polish market. However, this rule does not pertain to obligatory vaccinations. An advertisement may not be misleading and it should objectively inform about the properties of the product. It cannot target children. Publicly known persons, scientists, pharmacists or doctors (or persons appearing to have such professional background) cannot advertise drugs. Companies are not allowed to offer any benefits in return for purchasing drugs. Moreover, the advertisement must not suggest that by taking the advertised medication a person can avoid seeing a physician; that one’s health will deteriorate by not taking it (excluding the mandatory vaccinations); that the medicinal product is a foodstuff, cosmetic or other consumer product; or that the efficacy or safety of the medicinal product results from its natural origin. The allowed content of the advertisement is also regulated. For example, an advertisement cannot use inappropriate, disturbing or misleading terms for graphically depicted lesions, injuries or effects of the medicinal product on the human body.
Advertising of medicinal products to persons qualified to prescribe or market them, e.g. physicians, nurses, pharmacists, must contain clinical information (in line with the Summary of Product Characteristics) and information on the public availability (i.e. OTC or prescription). In case of publicly reimbursed products, this should also include information on the official retail price and maximum patient co-payment. Free marketing samples may be supplied only to persons qualified to prescribe but this excludes narcotic or psychotropic substances. The number of free samples is limited to no more than five packages of the product per year and the value of any marketing and promotional items cannot exceed PLN 100. Apart from this, it is prohibited to provide, offer or promise any material benefits (including gifts, prizes and excursions) or organize and finance promotional meetings for medicinal products, in which hospitality is incommensurate with the main purpose of the meeting.
Regulation of wholesalers and pharmacies
Entry requirements for new pharmacies
A permission issued by the Chief Pharmaceutical Inspector and an entry into the National Register of Manufacturers, Importers and Distributors of Active Substances (maintained by the Chief Pharmaceutical Inspector) are needed in order to undertake entrepreneurial activities related to the production, import or distribution of medicinal products and active pharmaceutical substances. Rules on Good Manufacturing Practice and Good Distribution Practice defined in the Act on the Pharmaceutical Law must be observed. Entities purchasing and selling medicinal products (with the exception of wholesale trade) must be registered in the National Register of Intermediaries in Trade in Medicinal Products (also maintained by the Chief Pharmaceutical Inspector). In order to operate, pharmaceutical wholesalers need an authorization to operate from the Chief Pharmaceutical Inspector and have to be registered in the Register of Authorizations for Pharmaceutical Wholesale. They must observe regulations on the wholesale trade of medicines, including the Good Distribution Practices, as set out in the Act on the Pharmaceutical Law.
There are several categories of pharmaceutical retail outlets in Poland. Outpatient pharmacies need a valid authorization from the Voivodeship Pharmaceutical Inspector in order to operate. The authorization can be granted if the number of inhabitants per one outpatient pharmacy is at least 3000 and the distance from the planned location of the pharmacy to the nearest pharmacy is at least 500 metres (these rules can be waived in individual cases by the Minister of Health). A single authorization holder cannot operate a pharmaceutical wholesale point or run or control more than four outpatient pharmacies (or more than 1% of outpatient pharmacies) in the voivodeship. The head of pharmacy has to be a pharmacist (with a master degree in pharmacy) with at least 5 years of experience of working in a pharmacy, or 3 years of experience if they have a postgraduate specialization in pharmacy, and must be present within the opening hours. One pharmacist can be the head of only one pharmacy. Only pharmacists and pharmaceutical technicians may be employed in a pharmacy. Moreover, since 2017 only a pharmacist can obtain an authorization to run a pharmacy (the goal was to reduce the number of pharmaceutical chains) (Kawalec & Kowalska-Bobko, 2018).
Opening times of outpatient pharmacies should be adapted to the needs of the population – they are determined by the county councils in consultations with the territorial self-government units and the professional self-government of pharmacists. Access to pharmaceuticals should be ensured at night, during weekends and public holidays. Outpatient pharmacies must stock medicinal products, foodstuffs for special nutritional use and medical devices in the quantities and assortment which are necessary to meet the health needs of the local population.
Generic substitution
Generic substitution is well developed in Poland. It has been stimulated by the changes to the pharmaceutical pricing and reimbursement introduced in 2012 which led to a decrease of their prices: according to these changes the first generic equivalent applying for reimbursement must be 25% cheaper than the branded product on the reimbursement list and any products subsequently added to the reimbursement list cannot be more expensive than the current reimbursement limit.
Mail order/Internet pharmacies
The mail order or Internet trade of medicines is allowed but only for OTC medicines and with the exception of medicinal products that can only be dispensed to patients of certain age. Only outpatient pharmacies and pharmacy outlets can sell medicinal products by mail order or Internet.
Regulation of counterfeit drugs
According to the Act on the Pharmaceutical Law, a falsified medicinal product is a medicine that:
- does not meet the established quality requirements for medicines;
- has been produced illegally without the knowledge of the manufacturer;
- has been produced without the consent of the State Pharmaceutical Inspection.
In 2007, the Minister of Health appointed the Team for Counterfeiting and Illicit Trade in Medicinal Products and Other Counterfeit Medicinal Products Fulfilling the Criteria of a Medicinal Product. The main tasks of this team include minimizing the extent of trade in counterfeit drugs and trade in unauthorized sales points, provision of information about falsified medicinal products, and conducting educational campaigns about the risks associated with purchasing medicines in unauthorized places (MZ, 2018e). Controls of medicines in pharmacies, pharmaceutical outlets and pharmaceutical wholesalers, which have been performed so far by national laboratories cooperating with the State Pharmaceutical Inspection, have not found falsified medicines in these facilities (GIF, 2018b; GIF/rynekaptek.pl, 2018).
Pricing of prescription pharmaceuticals
Profit control scheme
A clawback system, understood as a process by which the relevant authority can recoup some of the profits made by pharmacies via their dispensing margins, is not applied in Poland. However, a clawback on excessive reimbursement expenditure has been applied since 2012 at the level of the health system (see section 3.2). If the set cap is exceeded, pharmaceutical companies are expected to pay back 50% of excessive expenditure. So far, the cap has not been reached.
Reference pricing
Both external and internal reference pricing is used in the reimbursement of pharmaceuticals in Poland. External reference pricing takes into consideration prices of medicines from all EU and EFTA Member States. Within the internal reference pricing system, reference groups are established for medicinal products having the same international name or other international names but a similar therapeutic effect and mechanism of action. Medicines in the same reference group should have the same indications or uses in which they are reimbursed and similar effectiveness. The reference price in a given reference group of medicines used in outpatient care is based on the highest among the lowest wholesale prices for a defined daily dose (DDD) of a medicine whose volume turnover crosses the 15% threshold of the total volume turnover in that reference group. Reference prices are periodically updated. Different rules apply for establishing reference prices for medicines used in chemotherapy and within pharmaceutical programmes.
Direct price controls
Direct price controls are extensively used in pharmaceutical pricing and reimbursement in Poland. All reimbursed medicines are assigned officially established prices, which are set by the Minister of Health for 2–3 years.
Composition of prices
Prices are negotiated between the Minister of Health and the producers and are published as statutory pricing decisions of the Minister of Health. Statutory prices of medicines within inpatient care are interpreted as maximum prices and purchasers may negotiate lower prices in the purchasing process. Statutory reference prices are also applied to outpatient medicines and set the reimbursement limit above which hospitals cannot be reimbursed by the NFZ. This prevents manufacturers or wholesalers from proposing prices that are higher than reference prices. Prices of reimbursed outpatient medicines are also published as statutory pricing decision of the Minister of Health. They are determined as follows: the official net selling price (ex-factory price) is negotiated between the Minister of Health and the MAH; to this price the wholesaler adds the official margin (since 2012 5% of the official selling price; down from 9% previously) and the pharmacy adds the official retail margin, which depends on the medicine’s wholesale price. VAT (8%) is added on top. Since 2012, the pharmacies’ margin has been linked to the price limit established for a particular group of medicines (instead of the price of a particular medicine) in order to remove the incentive to sell more expensive medicines from the same group.
Prices and wholesale and retail margins of medicines that are not reimbursed can be set freely by the pharmaceutical wholesalers and retailers.
Public reimbursement of pharmaceuticals
Reimbursement of medicines, foodstuffs for special nutritional use and medical devices in Poland is based on positive lists (see section 2.4.3). Reimbursement decisions are taken by the Minister of Health and are based on recommendations of the Economic Commission and the president of AOTMiT and a number of criteria, including efficacy, safety, budget impact, and so on. As of January 2018, the following items were included in these positive lists.
- Lists A1–A3: medicines (4263 items identified with the European Article Number (EAN) or equivalent code), foodstuffs for special nutritional use (72 items) and medical devices (567 items) available in pharmacies on prescription; these items are available to patients free of charge or against cost-sharing (see Table3.8) up to an appropriate reimbursement limit.
- List B: medicines (usually new and innovative and usually also expensive) that are covered by special pharmaceutical programmes (programy lekowe) and are exempted from cost-sharing (379 items).
- List C: medicines used in chemotherapy that are exempted from cost-sharing (466 items).
- List D: medicines that are available free of charge to persons aged 75+ (1656 items).
Table3.8
Medicines provided as part of inpatient treatment are financed by the NFZ and are provided free of charge. Prices of some medicines that are provided as part of guaranteed health care services financed by the NFZ are set by the Minister of Health through administrative decisions and are not published in the positive lists.
On 28 September 2023, an amendment to the Pharmaceutical Law Act was introduced to strengthen the provisions of the Pharmacy for Pharmacists Act (2017). The amendment is colloquially referred to as the “Pharmacy for Pharmacists 2.0 amendment” or AdA 2.0.
According to the amendment, only a pharmacist can own a pharmacy. In addition, the pharmacist will not be able to hold shares in companies that control pharmacies and will not be able to establish a chain with more than four pharmacies under competition and consumer protection laws. In addition, a member of the governing body of a company authorized to operate a pharmaceutical wholesaler or a medicinal products intermediary will similarly not be able to take over an entity running a retail pharmacy. Finally, if an unauthorized person (that is, non-pharmacist) takes over a pharmacy or pharmacy chain, the pharmaceutical inspector can revoke their authorization and prevent them from doing so.
The aim of these regulations is to prevent entities operating as pharmacy chains or franchises from taking over companies operating pharmacies. The Pharmacy for the Pharmacist Act introduced in 2017 made it impossible for those entities to open new pharmacies. As a result, they began to increase their market shares by taking over and buying smaller pharmacies. AdA 2.0 contains provisions to close this loophole. It also gives the pharmaceutical inspectorate the right to revoke licenses and impose a fine of up to PLN 2 million on entities that violate these regulations.
Although AdA 2.0 only refers to the pharmacy retail market, it also introduces changes for pharmaceutical wholesalers. Moreover, it contains a range of sanctions for violating the regulations it introduces. These include both financial penalties and administrative sanctions such as the withdrawal of the license to operate a pharmacy.
The amendment also includes a provision that reduces the frequency of pharmaceutical inspections in wholesalers and entities that operate wholesalers. Currently, such inspections must be carried out every three years. According to the new regulation, the Chief Pharmaceutical Inspectorate will repeat inspections every five years. Some pharmacists view this is as a purely formal and long-awaited change. It results from the insufficient resources of the Chief Pharmaceutical Inspectorate to inspect wholesalers.