-
05 December 2025 | Country Update
New reform on patients sent abroad for cross-border care
2.7. Regulation
The government exercises its regulatory role at the national level through health legislation, as well as through the HIO and SHSO. In some cases, other ministries, such as the MoF and the Ministry of Education, or the Parliament may also have some regulatory function in the field of health. The EU has a regulatory role through its directives, which Cyprus has incorporated into national legislation, in many issues related to public health, such as professional qualifications of health personnel, quality of water for human consumption, medical equipment and pharmaceuticals.The Health Insurance Organization (HIO) has introduced a structural reform regarding the management of patients sent abroad for healthcare services. This reform represents a significant revision of the governance and operational framework for cross-border healthcare within the GHS (ΓεΣΥ), aiming to streamline processes, enhance clarity and ensure efficient management of cases requiring treatment abroad.
As of 2 October 2025, the HIO is responsible for processing and approving all overseas referral requests for GHS beneficiaries when the required medical service falls within the GHS benefits package and cannot be provided in Cyprus due to limitations in expertise, technology or clinical conditions. Requests for non-GHS beneficiaries will continue to be managed centrally by the Ministry of Health.
The HIO has also clarified that certain categories of services do not fall under its responsibility. These include interventions not sufficiently tested or recognized by conventional medicine, treatments in an experimental stage, requests relating solely to drug administration, non-conventional medical practices, telemedicine services and retroactive requests for services already received. Applications in these categories will continue to be overseen and processed by the Ministry of Health.
To ensure timely processing, the HIO has introduced a classification system with specified assessment timelines. Regular cases—those without strict time constraints—are evaluated within 15 days. Urgent cases, requiring accelerated management, are processed within 7 days, with the HIO typically completing assessments within 4 days. Life-threatening cases, requiring immediate attention, are processed within 2 days, with evaluations usually finalized within 24 hours.
The HIO has established a list of collaborating international hospitals for the provision of approved services abroad. However, beneficiaries retain the right to select a hospital outside this list. In such cases, and upon approval of the request, the beneficiary and their treating physician must make all necessary arrangements related to scheduling and admission. Coverage remains subject to the terms of the approved referral.
2.7.1. Regulation and governance of third parties
The HIO and the SHSO are the third-party payers within the new health care system, as are the private insurance companies which provide VHI coverage outside and beyond the GeSY. The HIO and the SHSO are public legal entities, governed by boards of directors, with relatively high autonomy in their activity.
The HIO, responsible for the implementation and management of the new system, is managed by a 13-member board of directors. Similarly, the SHSO, which is responsible for the organization and operation of public hospitals and health centres, is managed by a nine-member board of directors. In both cases, the members of the board of directors are appointed by the Council of Ministers, following a proposal by the MoH. For more information about HIO and SHSO see Chapter 6.
Finally, private insurance companies are under the supervision and control of the Insurance Companies Audit Service of the MoF, with their activity regulated by the Law 38(I) of 2016 and its amendments (The Insurance and Reinsurance Business and Other Related Issues Law of 2016).
2.7.2. Regulation and governance of provision
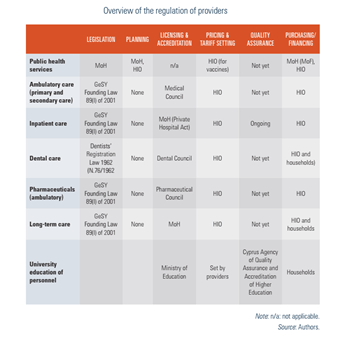
Responsibility for the regulation and governance of provision rests primarily with the HIO and secondarily with the SHSO. The HIO has the overall regulatory role for the system at the national level, while at a lower level, this role for public hospitals and health centres rests with the SHSO. In practice, as expected, the GeSY as a newly introduced system, has gaps and shortcomings, especially in the fields of regulation and governance. For example, there are currently no statutory mechanisms to ensure that providers achieve minimum standards of competence, governance and management arrangements or quality assurance systems in place to ensure and monitor the quality of care provided.
Nevertheless, efforts are being made in the right direction, either by developing and implementing clinical guidelines or by looking for ways that will lead to the improvement of the quality of services provided and the effectiveness of medical procedures. A relevant example is the ongoing dialogue between the HIO and contracted public and private hospitals for a different way of reimbursement, incorporating quality and efficiency indicators (see more details in Section 7.4). Also worth mentioning is the SHSO’s recent agreement with a Canadian organization for the accreditation of public hospitals, starting with the Nicosia General Hospital and the Makarion Hospital for Children and Women. Finally, the establishment of a National Centre on Clinical Documentation, the accreditation of provided health care services, and the upgrading of medical technology and medical devices in hospitals, provided by the Cyprus National Recovery and Resilience Plan 2021–2026, is expected to have significant added value in the quality and effectiveness of services provided. More actions will nevertheless be needed in terms of governance and regulation for better quality and effectiveness of services provided.
An overview of the regulation of providers is presented in Table2.1.
Table2.1
2.7.3. Regulation of services and goods
BASIC BENEFIT PACKAGE
The Founding Law 89(I)/2001 of the GeSY and its amending Law 74(I) (Law of 74(I)/2017) explicitly define the medical and other health care services that are provided and reimbursed within the new system. The procedures and the relevant details, including the amount and the method of reimbursement, are then reflected in internal regulations and decisions issued by the HIO after discussion and consultation with professional associations or representatives of health care providers, with the consent of the MoH.
In addition, an advisory Scientific Committee of doctors supports the HIO’s Board on issues of protocols, restrictions on medical procedures and best practices. Following the opinion of the above-mentioned medical Committee, the Board may reject the provision of health care services of limited or uncertain effectiveness, or health care services whose cost is too high and may jeopardize the sustainability of the system. The cost of any health care service is always adjusted accordingly so that the total cost does not exceed the expenditure set out in the global budget for the health care group to which it corresponds.
Under the GeSY, the responsibilities of the MoH regarding the reimbursement of pharmaceuticals have shifted to the HIO. Following the implementation of the new system, any medicinal product manufactured or imported, for which a marketing authorization is in force in accordance with the provisions of the “Medicinal Products for Human Use (Quality Control, Supply and Prices) Law” (70(I)/2001) can be included in the list of reimbursed pharmaceutical products by the HIO, provided that a positive decision is granted by the Council of the HIO following the relevant scientific committee’s recommendations. The criteria used as a basis for decision-making is determined by HIO internal regulations. Clinical evidence with respect to comparative effectiveness, evidence-based clinical guidelines and existing health technology assessments (HTAs) performed by other European decision-making bodies are deemed important in the process for consideration of drugs for reimbursement. It is worth noting that the decision for the inclusion of pharmaceutical products by the HIO Council is further shaped by the recommendations of the Drug Compensation Advisory Committee appointed by the HIO Council, which advises the HIO Council on compensation for pharmaceutical products that are or will be included in the list of pharmaceutical products.
By law, the role of the MoH in reimbursement decisions is limited to public health issues and the exceptional event of emergencies, such as the COVID-19 pandemic, in which case the cost of vaccines and other pharmaceutical products is borne by the MoH. However, the MoH is still responsible for covering the expenses for new and expensive pharmaceutical products due to significant delays in the HIO processes for concluding timely agreements with the marketing authorization holders.
HEALTH TECHNOLOGY ASSESSMENT
Despite extensive health care reforms being put in place, little progress has been made in terms of developing HTA capacity. Establishing HTA capacity was a specific engagement under the agreed international financial assistance package covered by the Memorandum of Understanding on Specific Economic Policy Conditionality signed in 2013.
In this context, the Support Group for Cyprus financed consultancy work in 2014 by the London School of Hygiene & Tropical Medicine to support the Cypriot authorities in their efforts to establish HTA capacity, although ultimately, implementation of this initiative did not proceed.
2.7.4. Regulation and governance of pharmaceuticals
Ensuring the quality, efficacy, safety and pricing of pharmaceuticals is the responsibility of the Department of Pharmaceutical Services (DPhS), which falls under the MoH. The DPhS acts as the Secretariat of the Drugs Council, providing evaluation services at pre- and post-approval level, as well as inspection and control/supervision services. The activities of the DPhS regarding the licensing and marketing of medicinal products for human use are regulated in accordance with the “Medicinal Products for Human Use (Control of Quality, Supply and Pricing) Law of 2001 (70(I)/2001)”, which is administered by the Drugs Council. This Law is in full harmonization with Community Directive 2001/83/EC. Consequently, medicinal products are marketed in Cyprus either through national procedures, or through cooperation with EU Member States for centralized, decentralized and mutual recognition procedures.
Post-marketing monitoring of the use and safety of medicinal products rests with the pharmacovigilance and clinical trials sectors of the DPhS, which manages the pharmacovigilance system in accordance with Legislation 70(I)/2001 based on the relevant European guidelines.
Patent protection is harmonized with EU legislation under the European Patent Convention and guarantees market protection for original pharmaceuticals including the provision for Supplementary Protection Certificates for patented pharmaceuticals. Finally, it should be noted that the debate on the bill for the establishment of a National Pharmaceutical Authority for the licensing and marketing of medicines is still pending.
REGULATION OF WHOLESALERS AND PHARMACIES
The inspection of all pharmacies, including in the private sector, is carried out by the Good Manufacturing and Distribution of Pharmaceutical Products (GMDP) Inspectorate of the MoH. According to this Law, no person other than a person registered as a pharmacist can operate, either for themselves or on behalf of another, a pharmacy or perform the duties of a hospital pharmacist unless the pharmacies are registered with the Pharmacy Board, which is also responsible for the registration of pharmacists. No person may be the owner of more than one pharmacy, or be the holder or beneficiary of more than 51% of the share capital of more than one company operating a pharmacy business. Neither Internet nor mail-order pharmacies are allowed. Although there are no geographical restrictions in operating a pharmacy, most are concentrated in urban areas and the majority of them have joined the GeSY.
The Cyprus Medicines Verification Organization was established in February 2017 as a non-profit legal entity to ensure the implementation of a national medicines verification system that complies with the Falsified Medicines Directive and the Delegated Regulation. This verification system enables health professionals to check the authenticity of medication before these are dispensed to patients, in order to reduce the risk of falsified medicines entering the legal distribution chain of pharmaceuticals.
Within the GeSY context, pharmacists are allowed to dispense the cheapest medicinal product of the same active substance and pharmaceutical form (generic substitution).
PRICING PRESCRIPTION PHARMACEUTICALS
The Medicinal Product Pricing Sector of the DPhS handles all issues and policies governing the pricing of medicinal products for human use upon receipt of a market authorization licence and is responsible for the annual update of the price list of medicinal products and the evaluation of the pricing policy (every two years) in accordance with the provisions of the Ministerial Notifications. The price of a pharmaceutical must be set within 90 days of receipt of a fully completed application; otherwise, the applicant may freely set the price.
The pricing policy of Cyprus is included in the provisions of the “Medicines for Human Use (Control of Quality, Supply and Pricing) Law 70(I)/2001”, as well as in the provisions of the relevant Ministerial Notices and Decrees issued by the MoH.
The main role of the pricing policy is to determine the prices of medicinal products (prescription medicines) by comparing the prices in reference countries, or by calculation, up to 80% of the price of the existing reference medicines in the price list in the case of prescription medicines of the same active substance (generics). The reference price is the average wholesale price of a particular medicinal product in the reference countries. For the calculation of the reference price, the wholesale prices in all 10 reference countries are examined and the lowest price from the group of expensive countries (Austria, Germany, Denmark), the two lowest prices from the group of medium countries (Belgium, Spain, Italy, Sweden) and the lowest price from the group of least expensive countries (France, Greece, Portugal) will be taken into account.
Special provisions for imported prescription medicinal products with a maximum wholesale price equal to or less than €6 and annual sales volume less than €25 000 are included in the legislation to prevent radical reductions in the prices of cheap medicines and to ensure their adequate supply in the market. The maximum wholesale selling price of imported prescription medicinal products is determined using the reference price, adding 3% for import costs.
The wholesale price of prescription medicines of the same active substance is calculated to equal 80% of the wholesale price of the respective originals (same form, packaging and content). The price of over-the-counter drugs is completely liberalized and decided between the supplier and the pharmacy.
2.7.5. Regulation of medical devices and aids
The MPHS in the MoH is the Cyprus Authority for Medical Devices which is the competent authority responsible for ensuring the safety, performance, and compliance of medical devices within the Republic of Cyprus. It operates under the framework of EU legislation, specifically the EU Medical Device Regulation (MDR) 2017/745 and In Vitro Diagnostic Medical Device Regulation (IVDR) 2017/746, as well as applicable Cypriot national laws.
The Authority monitors medical devices on the market to ensure compliance with EU and Cypriot regulations, verifying CE markings and proper labelling. It conducts inspections of manufacturers, importers, distributors, and health care facilities to ensure devices meet regulatory and safety standards. The Authority also manages a centralized database for the registration of medical devices placed on the Cypriot market.
In addition, it investigates reports of adverse events or malfunctions involving medical devices through its vigilance system, taking corrective actions to protect patient safety. It ensures compliance with legal requirements and has the power to impose penalties or restrictions when violations occur. Furthermore, the Authority provides information and guidance to businesses and health care providers regarding regulatory requirements and compliance.
By aligning its activities with European standards and conducting rigorous inspections and market inspections, the Cyprus Medical Devices Competent Authority plays a critical role in protecting public health and maintaining trust in medical technology.